Corynebacterium glutamicum ArnR controls expression of nitrate reductase operon narKGHJI and nitric oxide (NO)-detoxifying enzyme gene hmp in an NO-responsive manner
- PMID: 24142248
- PMCID: PMC3911137
- DOI: 10.1128/JB.01004-13
Corynebacterium glutamicum ArnR controls expression of nitrate reductase operon narKGHJI and nitric oxide (NO)-detoxifying enzyme gene hmp in an NO-responsive manner
Abstract
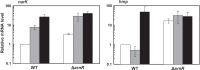
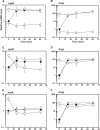
Corynebacterium glutamicum ArnR is a novel transcriptional regulator that represses expression of the nitrate reductase operon narKGHJI and the nitric oxide (NO)-detoxifying flavohemoglobin gene hmp under aerobic conditions. In a previous study, we showed that ArnR-mediated repression is relieved during anaerobic nitrate respiration, but we could not pinpoint the specific signal that ArnR senses. In this study, we show that in the absence of nitrate, ArnR-mediated repression is maintained under anaerobic conditions. The derepression in response to nitrate is eliminated by disruption of narG, suggesting that ArnR senses nitrate derivatives generated during nitrate respiration. Specifically, the hmp gene is upregulated in the presence of nitrite or nitric oxide (NO) in an ArnR-dependent manner, although the response of narK appears to be greatly affected by ArnR-independent regulation. In vitro binding of ArnR to the narK and hmp promoter regions is more strongly inhibited by NO than by nitrite. We previously showed that the UV-visible spectrum of ArnR is typical of a Fe-S cluster-containing protein. Site-directed mutagenesis of each of three cysteine residues, which are possibly involved in coordination of the cofactor in the ArnR protein, results in loss of the binding of this protein to its target promoters in vitro and eliminates the repression of the target genes in vivo under aerobic conditions. These observations suggest that the cofactor coordinated by these three cysteine residues in the ArnR protein plays a critical role in the NO-responsive expression of the narKGHJI operon and the hmp gene.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

