SMU.746-SMU.747, a putative membrane permease complex, is involved in aciduricity, acidogenesis, and biofilm formation in Streptococcus mutans
- PMID: 24142257
- PMCID: PMC3911122
- DOI: 10.1128/JB.00960-13
SMU.746-SMU.747, a putative membrane permease complex, is involved in aciduricity, acidogenesis, and biofilm formation in Streptococcus mutans
Abstract
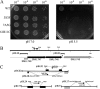
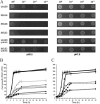
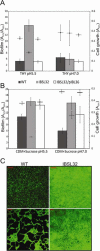
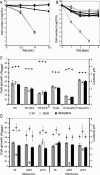
Dental caries induced by Streptococcus mutans is one of the most prevalent chronic infectious diseases worldwide. The pathogenicity of S. mutans relies on the bacterium's ability to colonize tooth surfaces and survive a strongly acidic environment. We performed an ISS1 transposon mutagenesis to screen for acid-sensitive mutants of S. mutans and identified an SMU.746-SMU.747 gene cluster that is needed for aciduricity. SMU.746 and SMU.747 appear to be organized in an operon and encode a putative membrane-associated permease. SMU.746- and SMU.747-deficient mutants showed a reduced ability to grow in acidified medium. However, the short-term or long-term acid survival capacity and F1F0 ATPase activity remained unaffected in the mutants. Furthermore, deletion of both genes did not change cell membrane permeability and the oxidative and heat stress responses. Growth was severely affected even with slight acidification of the defined medium (pH 6.5). The ability of the mutant strain to acidify the defined medium during growth in the presence of glucose and sucrose was significantly reduced, although the glycolysis rate was only slightly affected. Surprisingly, deletion of the SMU.746-SMU.747 genes triggered increased biofilm formation in low-pH medium. The observed effects were more striking in a chemically defined medium. We speculate that the SMU.746-SMU.747 complex is responsible for amino acid transport, and we discuss its possible role in colonization and survival in the oral environment.
Figures

Similar articles
-
Identification and functional analysis of the L-ascorbate-specific enzyme II complex of the phosphotransferase system in Streptococcus mutans.BMC Microbiol. 2016 Mar 22;16:51. doi: 10.1186/s12866-016-0668-9. BMC Microbiol. 2016. PMID: 27001419 Free PMC article.
-
Glycosyltransferase-Mediated Biofilm Matrix Dynamics and Virulence of Streptococcus mutans.Appl Environ Microbiol. 2019 Feb 20;85(5):e02247-18. doi: 10.1128/AEM.02247-18. Print 2019 Mar 1. Appl Environ Microbiol. 2019. PMID: 30578260 Free PMC article.
-
SMU.940 regulates dextran-dependent aggregation and biofilm formation in Streptococcus mutans.Mol Oral Microbiol. 2018 Feb;33(1):47-58. doi: 10.1111/omi.12196. Epub 2017 Oct 11. Mol Oral Microbiol. 2018. PMID: 28845576
-
Deciphering the role of SMU.1147 in peptide-mediated signaling and competence in Streptococcus mutans.Microbiol Spectr. 2025 Apr;13(4):e0291724. doi: 10.1128/spectrum.02917-24. Epub 2025 Mar 5. Microbiol Spectr. 2025. PMID: 40042332 Free PMC article.
-
Core-gene-encoded peptide regulating virulence-associated traits in Streptococcus mutans.J Bacteriol. 2013 Jun;195(12):2912-20. doi: 10.1128/JB.00189-13. Epub 2013 Apr 19. J Bacteriol. 2013. PMID: 23603743 Free PMC article.
Cited by
-
Acid-adaptive mechanisms of Streptococcus mutans-the more we know, the more we don't.Mol Oral Microbiol. 2017 Apr;32(2):107-117. doi: 10.1111/omi.12162. Epub 2016 Jun 21. Mol Oral Microbiol. 2017. PMID: 27115703 Free PMC article. Review. No abstract available.
-
Identification of an Efflux Transporter LmrB Regulating Stress Response and Extracellular Polysaccharide Synthesis in Streptococcus mutans.Front Microbiol. 2017 Jun 8;8:962. doi: 10.3389/fmicb.2017.00962. eCollection 2017. Front Microbiol. 2017. PMID: 28642736 Free PMC article.
-
[Role of SMU.2055 gene in regulating acid resistance of Streptococcus mutans UA159].Nan Fang Yi Ke Da Xue Xue Bao. 2018 Feb 20;38(2):198-204. doi: 10.3969/j.issn.1673-4254.2018.02.13. Nan Fang Yi Ke Da Xue Xue Bao. 2018. PMID: 29502060 Free PMC article. Chinese.
-
Effect of Different Glucose Concentrations on Small RNA Levels and Adherence of Streptococcus mutans.Curr Microbiol. 2019 Nov;76(11):1238-1246. doi: 10.1007/s00284-019-01745-1. Epub 2019 Aug 3. Curr Microbiol. 2019. PMID: 31377819
-
Transposon mutagenesis in oral streptococcus.J Oral Microbiol. 2022 Jul 24;14(1):2104951. doi: 10.1080/20002297.2022.2104951. eCollection 2022. J Oral Microbiol. 2022. PMID: 35903085 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

