Naproxen with or without an antiemetic for acute migraine headaches in adults
- PMID: 24142263
- PMCID: PMC6540401
- DOI: 10.1002/14651858.CD009455.pub2
Naproxen with or without an antiemetic for acute migraine headaches in adults
Abstract
Background: Migraine is a common, disabling condition and a burden for the individual, health services, and society. Many sufferers choose not to, or are unable to, seek professional help and rely on over-the-counter analgesics. Naproxen is a non-steroidal anti-inflammatory drug (NSAID); its efficacy in acute migraine has not been established by systematic reviews. Co-therapy with an antiemetic should help to reduce the nausea and vomiting commonly associated with migraine headaches.
Objectives: To determine the efficacy and tolerability of naproxen, alone or in combination with an antiemetic, compared with placebo and other active interventions in the treatment of acute migraine headaches in adults.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library, MEDLINE, EMBASE, and the Oxford Pain Relief Database, together with two online databases (www.gsk-clinicalstudyregister.com and www.clinicaltrials.gov) and reference lists, for studies to 22 May 2013.
Selection criteria: We included randomised, double-blind, placebo- or active-controlled studies, with at least 10 participants per treatment arm, using naproxen alone or with an antiemetic to treat a migraine headache episode.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. We used numbers of participants achieving each outcome to calculate risk ratios and numbers needed to treat (NNT) or harm (NNH) compared with placebo or a different active treatment.
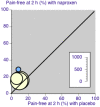
Main results: We included six studies using naproxen 275 mg, 500 mg, or 825 mg to treat attacks of moderate or severe pain intensity. Overall, 1241 participants took naproxen (275 mg to 825 mg), 229 took sumatriptan 50 mg, 173 took naratriptan 2.5 mg, and 1092 took placebo. No studies combined naproxen with an antiemetic. Studies using naproxen 275 mg provided no useable data for analysis.Naproxen (500 mg and 825 mg) was better than placebo for pain-free response and headache relief. At two hours, the NNT for pain-free response was 11 (17% response with naproxen, 8% with placebo; risk ratio 2.0 (95% CI 1.6 to 2.6), moderate quality) and for headache relief was 6.0 (45% response with naproxen, 29% with placebo; risk ratio 1.6 (1.4 to 1.8), moderate quality). The NNT for sustained pain-free response during the 24 hours post dose was 19 (12% response with naproxen, 6.7% with placebo), and for sustained headache relief during the 24 hours post dose was 8.3 (30% response with naproxen, 18% with placebo). Analysing only the lower dose of 500 mg of naproxen did not significantly change the results. Adverse events, which were mostly mild or moderate in severity and rarely led to withdrawal, were more common with naproxen than with placebo when the 500 mg and 825 mg doses were considered together, but not when the 500 mg dose was analysed alone.There were insufficient data for analysis of naproxen compared with sumatriptan, and no data suitable for analysis of naproxen compared with naratriptan.
Authors' conclusions: Naproxen is statistically superior to placebo in the treatment of acute migraine, but the NNT of 11 for pain-free response at two hours suggests that it is not a clinically useful treatment. Cochrane reviews examining other commonly used analgesics for acute migraine have reported better (lower) NNT results for the same outcome. Naproxen is not clinically useful as a stand-alone analgesic in acute migraine, as it is effective in fewer than 2 people in 10.
Conflict of interest statement
RAM has consulted for various pharmaceutical companies and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. RAM and SD have received research support from charities, government, and industry sources at various times. SL has no interests to declare.
Figures












Update of
References
References to studies included in this review
Brandes 2007 Study 1 {published data only}
-
- Brandes JL, Kudrow D, Stark SR, O'Carroll CP, Adelman JU, O'Donnell FJ, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA 2007;297(13):1443-54. [DOI: 10.1001/jama.297.13.1443 ] - PubMed
-
- Landy S, DeRossett SE, Rapoport A, Rothrock J, Ames MH, McDonald SA, et al. Two double-blind, multicenter, randomized, placebo-controlled, single-dose studies of sumatriptan/naproxen sodium in the acute treatment of migraine: function, productivity, and satisfaction outcomes. Medscape General Medicine 2007;9(2):53. - PMC - PubMed
Brandes 2007 Study 2 {published data only}
-
- Brandes JL, Kudrow D, Stark SR, O'Carroll CP, Adelman JU, O'Donnell FJ, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA 2007;297(13):1443-54. [DOI: 10.1001/jama.297.13.1443 ] - PubMed
-
- Landy S, DeRossett SE, Rapoport A, Rothrock J, Ames MH, McDonald SA, et al. Two double-blind, multicenter, randomized, placebo-controlled, single-dose studies of sumatriptan/naproxen sodium in the acute treatment of migraine: function, productivity, and satisfaction outcomes. Medscape General Medicine 2007;9(2):53. - PMC - PubMed
S2WA4003 {published data only}
-
- Anonymous. A randomized, double-blind, double-dummy, active-placebo controlled, parallel group evaluation of oral naratriptan (2.5mg) compared to oral naproxen sodium (275mg) on migraine-related quality of life. Result summary for S2WA4003. download.gsk-clinicalstudyregister.com/files/2014.pdf (accessed 19 September 2013).
S2WA4004 {published data only}
-
- Anonymous. A randomized, double-blind, double-dummy, active-placebo controlled, parallel group evaluation of oral naratriptan (2.5mg) compared to oral naproxen sodium (275mg) on migraine-related quality of life. Result summary for S2WA4004. download.gsk-clinicalstudyregister.com/files/2041.pdf (accessed 19 September 2013).
Smith 2005 {published data only}
Wentz 2008 {published data only}
-
- Wentz AL, Jimenez TB, Dixon RM, Aurora SK, Gold M, CXA20008 Study Investigators. A double-blind, randomized, placebo-controlled, single-dose study of thecyclooxygenase-2 inhibitor, GW406381, as a treatment for acute migraine. European Journal of Neurology 2008;15(4):420-7. [DOI: 10.1111/j.1468-1331.2008.02093.x] - DOI - PubMed
References to studies excluded from this review
Adis 2006 {published data only}
-
- Adis International Limited. Naproxen sodium/metoclopramide: metoclopramide/naproxen-sodium, MT 100, naproxen-sodium/metoclopramide. Drugs in R and D 2006;7(4):259-61.
Andersson 1989 {published data only}
-
- Andersson PG, Hinge HH, Johansen O, Andersen CU, Lademann A, Gøtzsche PC. Double-blind study of naproxen vs placebo in the treatment of acute migraine attacks. Cephalalgia 1989;9(1):29-32. - PubMed
Johnson 1985 {published data only}
-
- Johnson E, Ratcliffe D, Wilkinson M. Naproxen sodium in the treatment of migraine. Cephalalgia 1985;5(1):5-10. - PubMed
Krymchantowski 2005 {published data only}
Misra 2010 {published data only}
-
- Misra M, Sharma T, Kalra J, Goel D, Dhasmana DC. Comparative efficacy and tolerability of sumatriptan, ergotamine, naproxen and rizatriptan in moderate to severe acute attack of migraine. JK Science 2010;12(4):175-9.
NCT01726920 {published data only}
-
- Peron C. Efficacy and safety of a fixed-dose combination of naratriptan and naproxen in acute treatment of migraine (Copérnico). clinicaltrials.gov/show/NCT01726920 (accessed 19 September 2013). [ACH-NRP-03(04/12)]
Nestvold 1985 {published data only}
-
- Nestvold K, Kloster R, Partinen M, Sulkava R. Treatment of acute migraine attack: naproxen and placebo compared. Cephalalgia 1985;5(2):115-9. - PubMed
Nestvold 1986 {published data only}
-
- Nestvold K. Naproxen and naproxen sodium in acute migraine attacks. Cephalalgia 1986;6 Suppl 4:81-4. - PubMed
Pradalier 1985 {published data only}
-
- Pradalier A, Rancurel G, Dordain G, Verdure L, Rascol A, Dry J. Acute migraine attack therapy: comparison of naproxen sodium and an ergotamine tartrate compound. Cephalalgia 1985;5(2):107-13. - PubMed
Sargent 1988 {published data only}
-
- Sargent JD, Baumel B, Peters K, Diamond S, Saper JR, Eisner LS, et al. Aborting a migraine attack: naproxen sodium v ergotamine plus caffeine. Headache 1988;28(4):263-6. - PubMed
Smith 2007 {published data only}
Stronks 2003 {published data only}
Treves 1992 {published data only}
-
- Treves T, Streiffler M, Korczyn A. Naproxen sodium versus ergotamine tartrate in the treatment of acute migraine attacks. Headache 1992;32(6):280-2. - PubMed
Welch 1986 {published data only}
-
- Welch K. Naproxen sodium in the treatment of migraine. Cephalalgia 1986;6 Suppl 4:85-92. - PubMed
References to studies awaiting assessment
NCT01390324 {published data only}
-
- Peron C. Efficacy and safety of a fixed-dose combination of naratriptan and naproxen in acute treatment of migraine (Atenéia). clinicaltrials.gov/show/NCT01390324 (accessed 19 September 2013). [ACH-NRP-03(05/11)]
Additional references
Ayzenberg 2012
-
- Ayzenberg I, Katsarava Z, Sborowski A, Chernysh M, Osipova V, Tabeeva G, et al. The prevalence of primary headache disorders in Russia: a countrywide survey. Cephalalgia 2012;32(5):373-81. [DOI: 10.1177/0333102412438977 ] - PubMed
Bandolier 2000
-
- Anon. Naproxen for acute migraine, 2000. www.medicine.ox.ac.uk/bandolier/booth/Migraine/NapORacu.html (19 September 2013).
Bigal 2008
Bloudek 2012
-
- Bloudek LM, Stokes M, Buse DC, Wilcox TK, Lipton RB, Goadsby PJ, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). Journal of Headache and Pain 2012;13(5):361-78. [DOI: 10.1007/s10194-012-0460-7] - DOI - PMC - PubMed
Buse 2011
Collins 1997
-
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain 1997;72(1-2):95-7. - PubMed
Cook 1995
Derry 2009
Derry 2012
Derry 2013
Diamond 2007
-
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355-63. [DOI: 10.1111/j.1526-4610.2006.00631.x] - DOI - PubMed
Friedman 2005
Hazard 2009
Hernandez‐Diaz 2000
-
- Hernández-Diaz S, García Rodriguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding and perforation: an overview of epidemiological studies published in the 1990s. Archives of Internal Medicine 2000;160(14):2093-9. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 Available from www.cochrane-handbook.org.
IHS 1988
-
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1-96. - PubMed
IHS 2000
-
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765-86. - PubMed
IHS 2004
-
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1-160. - PubMed
IHS 2013
Jadad 1996a
-
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2-3):239-46. - PubMed
Jadad 1996b
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. - PubMed
Jakubowski 2007
Kirthi 2013
L'Abbé 1987
-
- L'Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine 1987;107(2):224-33. - PubMed
Law 2013
Leonardi 2005
Linde 2012
Lipton 1999
-
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy? Headache 1999;39 Suppl 2:S20-S26.
Lipton 2007
Lucas 2006
Martindale 2012
-
- Brayfield A. Martindale: the complete drug reference. www.medicinescomplete.com/mc/martindale/current/ (19 September 2013).
Moore 1998
Moore 2008
-
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15-24. [ISBN: 978-0-931092-69-5]
Moore 2010a
-
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374-9. [DOI: 10.1136/ard.2009.107805] - DOI - PMC - PubMed
Moore 2010b
Morris 1995
Munakata 2009
Pardutz 2010
Pozen 2005a
-
- Pozen Inc. MT 100 (naproxen sodium and metoclopramide hydrochloride) Tablets. Briefing document for peripheral and central nervous system drugs advisory committee meeting, 4 August 2005, 2005. www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4167B1_01_01-Pozen-Backgro... (accessed 19 September 2013).
Pozen 2005b
-
- Pozen Inc. MT100 for the treatment of migraine, 2005. www.fda.gov/ohrms/dockets/ac/05/briefing/ 2005-4167S1_01_POZEN-Presentation.ppt (accessed 17 April 2012).
Rabbie 2013
Radtke 2009
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ross‐Lee 1983
-
- Ross-Lee LM, Eadie MJ, Heazlewood V, Bochner F, Tyrer JH. Aspirin pharmacokinetics in migraine. The effect of metoclopramide. European Journal of Clinical Pharmacology 1983;24(6):777-85. - PubMed
Salazar‐Tortolero 2008
-
- Salazar-Tortolero G, Huertas-Campistol A, Vergez-Pinto L, Ramos-Brunet A, Lluch-López J. Metoclopramide as a painkiller for intense migraine headache in emergency departments [Metoclopramida como analgésicoen la cefalea migrañosa intensa en urgencias]. Revista de Neurologia 2008;47(10):506-8. [PMID: ] - PubMed
Steiner 2013
Stovner 2010
Suthisisang 2010
Tramer 1997
Victor 2010
-
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia 2010;30(9):1065-72. [DOI: 10.1177/0333102409355601 ] - PubMed
Volans 1974
Volans 1975
Vos 2012
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

