Airway smooth muscle in airway reactivity and remodeling: what have we learned?
- PMID: 24142517
- PMCID: PMC3882535
- DOI: 10.1152/ajplung.00259.2013
Airway smooth muscle in airway reactivity and remodeling: what have we learned?
Abstract
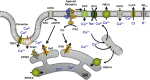
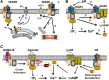
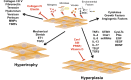
It is now established that airway smooth muscle (ASM) has roles in determining airway structure and function, well beyond that as the major contractile element. Indeed, changes in ASM function are central to the manifestation of allergic, inflammatory, and fibrotic airway diseases in both children and adults, as well as to airway responses to local and environmental exposures. Emerging evidence points to novel signaling mechanisms within ASM cells of different species that serve to control diverse features, including 1) [Ca(2+)]i contractility and relaxation, 2) cell proliferation and apoptosis, 3) production and modulation of extracellular components, and 4) release of pro- vs. anti-inflammatory mediators and factors that regulate immunity as well as the function of other airway cell types, such as epithelium, fibroblasts, and nerves. These diverse effects of ASM "activity" result in modulation of bronchoconstriction vs. bronchodilation relevant to airway hyperresponsiveness, airway thickening, and fibrosis that influence compliance. This perspective highlights recent discoveries that reveal the central role of ASM in this regard and helps set the stage for future research toward understanding the pathways regulating ASM and, in turn, the influence of ASM on airway structure and function. Such exploration is key to development of novel therapeutic strategies that influence the pathophysiology of diseases such as asthma, chronic obstructive pulmonary disease, and pulmonary fibrosis.
Keywords: asthma; bronchoconstriction; bronchodilation; calcium; development; extracellular matrix; inflammation; lung; proliferation.
Figures


References
-
- Algara-Suarez P, Mejia-Elizondo R, Sims SM, Saavedra-Alanis VM, Espinosa-Tanguma R. The 13 isoform of Na+-Ca2+ exchanger expressed in guinea pig tracheal smooth muscle is less sensitive to KB-R7943. J Physiol Biochem 66: 117–125, 2010 - PubMed
-
- Algara-Suarez P, Romero-Mendez C, Chrones T, Sanchez-Armass S, Meza U, Sims SM, Espinosa-Tanguma R. Functional coupling between the Na+/Ca2+ exchanger and nonselective cation channels during histamine stimulation in guinea pig tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol 293: L191–L198, 2007 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

