Methemoglobin-induced signaling and chemokine responses in human alveolar epithelial cells
- PMID: 24142518
- PMCID: PMC3920215
- DOI: 10.1152/ajplung.00066.2013
Methemoglobin-induced signaling and chemokine responses in human alveolar epithelial cells
Abstract
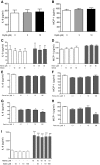
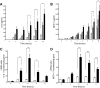
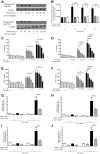
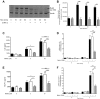
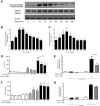
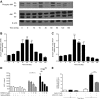
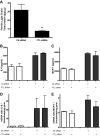
Diffuse alveolar hemorrhage is characterized by the presence of red blood cells and free hemoglobin in the alveoli and complicates a number of serious medical and surgical lung conditions including the pulmonary vasculitides and acute respiratory distress syndrome. In this study we investigated the hypothesis that exposure of human alveolar epithelial cells to hemoglobin and its breakdown products regulates chemokine release via iron- and oxidant-mediated activation of the transcription factor NF-κB. Methemoglobin alone stimulated the release of IL-8 and MCP-1 from A549 cells via activation of the NF-κB pathway; additionally, IL-8 required ERK activation and MCP-1 required JNK activation. Neither antioxidants nor iron chelators and knockdown of ferritin heavy and light chains affected these responses, indicating that iron and reactive oxygen species are not involved in the response of alveolar epithelial cells to methemoglobin. Incubation of primary cultures of human alveolar type 2 cells with methemoglobin resulted in a similar pattern of chemokine release and signaling pathway activation. In summary, we have shown for the first time that methemoglobin induced chemokine release from human lung epithelial cells independent of iron- and redox-mediated signaling involving the activation of the NF-κB and MAPK pathways. Decompartmentalization of hemoglobin may be a significant proinflammatory stimulus in a variety of lung diseases.
Keywords: alveolar epithelial cells; cytokines; methemoglobin.
Figures


Comment in
-
Thinking outside the cell: how cell-free hemoglobin can potentiate acute lung injury.Am J Physiol Lung Cell Mol Physiol. 2014 Feb;306(3):L231-2. doi: 10.1152/ajplung.00355.2013. Epub 2013 Dec 13. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24337924 Free PMC article. No abstract available.
References
-
- Alayash AI, Patel RP, Cashon RE. Redox reactions of hemoglobin and myoglobin: biological and toxicological implications. Antioxid Redox Signal 3: 313–327, 2001 - PubMed
-
- Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Jacob HS, Eaton JW, Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal 9: 2119–2137, 2007 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

