Pegylated liposomal doxorubicin for first-line treatment of epithelial ovarian cancer
- PMID: 24142521
- PMCID: PMC6457824
- DOI: 10.1002/14651858.CD010482.pub2
Pegylated liposomal doxorubicin for first-line treatment of epithelial ovarian cancer
Abstract
Background: Epithelial ovarian cancer (EOC) is often diagnosed at an advanced stage, requiring primary cytoreductive surgery and combination chemotherapy for its first-line management. Currently, the recommended standard first-line chemotherapy is platinum-based, usually consisting of carboplatin and paclitaxel (PAC/carbo). Pegylated liposomal doxorubicin (PLD) is an improved formulation of doxorubicin that is associated with fewer and less severe side effects than are seen with non-modified doxorubicin. In combination with carboplatin, PLD has recently been shown to improve progression-free survival compared with PAC/carbo in women with relapsed, platinum-sensitive EOC. It is therefore important to know whether any survival benefit can be attributed to PLD when it is used in the first-line setting.
Objectives: To evaluate the role of PLD, alone or in combination, in first-line chemotherapy for women with EOC.
Search methods: We searched The Cochrane Gynaecological Cancer Group's Trial Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE from January 1990 to February 2013. In addition, we searched online trial registries for ongoing trials and abstracts of studies presented at relevant scientific meetings from 2000 onwards.
Selection criteria: We included all randomised controlled trials (RCTs) that compared PLD alone or in combination with other agent/s (e.g. carboplatin) versus other agent/s for first-line chemotherapy in women with EOC who may or may not have undergone primary cytoreductive surgery.
Data collection and analysis: Two review authors independently selected trials, extracted data and assessed the risk of bias for each included trial. We obtained updated trial data when possible.
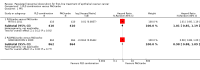
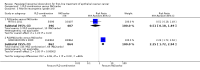
Main results: We included two large trials. One trial compared three-weekly PLD and carboplatin (PLD/carbo) with PAC/carbo. The other trial included four experimental arms, one of which was PLD plus PAC/carbo, that were compared with the standard PAC/carbo regimen. We did not combine results of these two trials in the meta-analysis. We considered the two studies to be at low risk of bias.For the comparison PLD/carbo versus PAC/carbo (820 women; stages Ic to IV), no statistically significant differences in progression-free survival (PFS) (hazard ratio [HR] 1.01, 95% confidence interval [CI] 0.85 to 1.19) or overall survival (OS) (HR 0.94, 95% CI 0.78 to 1.13) were noted between study arms. Severe anaemia (risk ratio [RR] 2.74, 95% CI 1.54 to 4.88) and thrombocytopenia (RR 8.09, 95% CI 3.93 to 16.67) were significantly more common with PLD/carbo, whereas alopecia (RR 0.09, 95% CI 0.06 to 0.14) and severe neurotoxicity (RR 0.09, 95% CI 0.01 to 0.66) were significantly more common with PAC/carbo. Quality of life scores were not significantly different.For the comparison PLD/PAC/carbo versus PAC/carbo (1726 women; stage III/IV), it is important to note that PLD was given for alternate cycles only (i.e. every 6 weeks). No statistically significant difference in PFS (HR 0.98, 95% CI 0.88 to 1.09) or OS (HR 0.95, 95% CI 0.84 to 1.08) between these two treatment arms was reported. However, women in the triplet arm experienced significantly more severe haematological adverse events (anaemia, thrombocytopenia, neutropenia and febrile neutropenia) compared with those given standard treatment.No RCTs evaluated single-agent PLD for first-line treatment of EOC.
Authors' conclusions: PLD/carbo is a reasonable alternative to PAC/carbo for the first-line treatment of EOC. Although three-weekly PLD/carbo may be associated with increased dose delays and discontinuations compared with the standard PAC/carbo regimen, it might be more acceptable to women who wish to avoid alopecia or those at high risk of neurotoxicity. No survival benefits appear to be associated with the alternating triplet regimen, and the additional toxicity associated with adding PLD to PAC/carbo limits further investigation. Further studies are needed to establish the safest, most effective PLD/carbo regimen for newly diagnosed disease.
Conflict of interest statement
None.
Figures
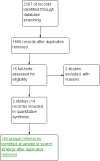
















Update of
- doi: 10.1002/14651858.CD010482
Similar articles
-
Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer.Cochrane Database Syst Rev. 2013 Jul 9;2013(7):CD006910. doi: 10.1002/14651858.CD006910.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2023 Jul 5;7:CD006910. doi: 10.1002/14651858.CD006910.pub3. PMID: 23835762 Free PMC article. Updated.
-
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD008766. doi: 10.1002/14651858.CD008766.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866378 Free PMC article.
-
Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer.Cochrane Database Syst Rev. 2022 Feb 16;2(2):CD007929. doi: 10.1002/14651858.CD007929.pub4. Cochrane Database Syst Rev. 2022. PMID: 35170751 Free PMC article.
-
Luteinising hormone releasing hormone (LHRH) agonists for the treatment of relapsed epithelial ovarian cancer.Cochrane Database Syst Rev. 2016 Jun 29;2016(6):CD011322. doi: 10.1002/14651858.CD011322.pub2. Cochrane Database Syst Rev. 2016. PMID: 27356090 Free PMC article.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
Cited by
-
The Growing Field of Nanomedicine and Its Relevance to Pharmacy Curricula.Am J Pharm Educ. 2021 Sep;85(8):8331. doi: 10.5688/ajpe8331. Epub 2021 Feb 24. Am J Pharm Educ. 2021. PMID: 34615620 Free PMC article.
-
High ERCC1 expression is associated with platinum-resistance, but not survival in patients with epithelial ovarian cancer.Oncol Lett. 2016 Aug;12(2):857-862. doi: 10.3892/ol.2016.4732. Epub 2016 Jun 15. Oncol Lett. 2016. PMID: 27446360 Free PMC article.
-
Predicting Response to Anthracyclines in Ovarian Cancer.Int J Environ Res Public Health. 2022 Apr 2;19(7):4260. doi: 10.3390/ijerph19074260. Int J Environ Res Public Health. 2022. PMID: 35409939 Free PMC article. Review.
-
Optimal management strategies for primary headache in the emergency department.CJEM. 2021 Nov;23(6):802-811. doi: 10.1007/s43678-021-00173-0. Epub 2021 Aug 14. CJEM. 2021. PMID: 34390484
-
Controlled Drug Release and Cytotoxicity Studies of Beta-Lapachone and Doxorubicin Loaded into Cyclodextrins Attached to a Polyethyleneimine Matrix.Int J Mol Sci. 2020 Aug 14;21(16):5832. doi: 10.3390/ijms21165832. Int J Mol Sci. 2020. PMID: 32823816 Free PMC article.
References
References to studies included in this review
GOG0182/ICON 5 2009 {published data only}
-
- Bookman MA. Erratum. Journal of Clinical Oncology 2009;27(13):2305.
-
- Bookman MA and the Gynecologic Cancer InterGroup (GCIG). GOG0182‐ICON5: 5‐arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG‐lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced‐stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I. 2006; Vol. 24, 18S (June 20 Suppl):5002.
-
- Bookman MA and the Gynecologic Cancer InterGroup (GCIG). GOG0182‐ICON5: 5‐arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG‐lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced‐stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma [abstract]. Journal of Clinical Oncology 2006;24(Suppl 18):A‐5002:256s.
-
- Bookman MA, Greer BE, Ozols RF. Optimal therapy of advanced ovarian cancer: carboplatin and paclitaxel vs. cisplatin and paclitaxel (GOG 158) and an update on GOG0 182‐ICON5. International Journal of Gynecological Cancer 2003;13(6):735‐40. - PubMed
MITO‐2 2011 {published data only}
-
- Pignata S, Scambia G, Ferrandina G, Savarese A, Sorio R, Breda E, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first‐line treatment for patients with ovarian cancer: the MITO‐2 randomized phase III trial. Journal of Clinical Oncology 2011;29(27):3628‐35. - PubMed
-
- Pignata S, Scambia G, Savarese A, Breda E, Scollo P, Vivo R, et al. Safety of a 3‐weekly schedule of carboplatin plus pegylated liposomal doxorubicin as first line chemotherapy in patients with ovarian cancer: preliminary results of the MITO‐2 randomized trial. BioMedCentral (BMC) Cancer 2006;6:202. http://www.biomedcentral.com/1471‐2407/6/202.. - PMC - PubMed
-
- Pignata S, Scambia G, Savarese A, Breda E, Sorio R, Pisano C, et al. Carboplatin and pegylated liposomal doxorubicin for advanced ovarian cancer: preliminary activity results of the MITO‐2 phase III trial. Oncology 2009;76(1):49‐54. - PubMed
-
- Pignata S, Scambia G, Savarese A, Breda E, Sorio R, Vernaglia Lombardi A, et al. Carboplatin plus paclitaxel versus carboplatin plus Stealth liposomal doxorubicin in patients with advanced ovarian cancer: preliminary activity results of the MITO‐2 randomized multicenter trial [Abstract]. Journal of Clinical Oncology; 2007 ASCO Annual Meeting. 2007; Vol. 25:18S(abstract 5532).
-
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Ferrandina G, et al. Carboplatin plus paclitaxel (CP) versus carboplatin plus stealth liposomal doxorubicin (CLD) in patients with advanced ovarian cancer (AOC): activity and safety results of the MITO‐2 randomized multicenter trial [abstract]. Journal of Clinical Oncology; 2009 ASCO Annual Meeting. 2009; Vol. 27:15S(Suppl; abstract LBA5508).
References to studies excluded from this review
Pontamianou 2005 {published data only}
-
- Potamianou A, Androulakis N, Papakotoulas P, Toufexi H, Latoufis C, Kouroussis C, et al. Sequential combination of paclitaxel‐carboplatin and paclitaxel‐liposomal doxorubicin as a first‐line treatment in patients with ovarian cancer. A multicenter phase II trial. Oncology 2005;69(4):348‐53. - PubMed
SWOG S9912 2009 {published data only}
-
- Smith HO, Moon J, Wilczynski SP, Tiersten AD, Hannigan EV, Robinson WR, et al. Southwest Oncology Group Trial S9912: intraperitoneal cisplatin and paclitaxel plus intravenous paclitaxel and pegylated liposomal doxorubicin as primary chemotherapy of small‐volume residual stage III ovarian cancer. Gynecologic Oncology 2009;114(2):206‐9. - PMC - PubMed
Additional references
A'Hern 1995
-
- A'Hern R, Gore ME. The impact of doxorubicin on survival in advanced ovarian cancer. Journal of Clinical Oncology 1995;13:726‐32. - PubMed
Aaronson 1993
-
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365‐76. - PubMed
Burger 2011
-
- Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, et al. for the Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England Journal of Medicine 2011;365:2473‐83. - PubMed
CALYPSO 2010
-
- Pujade‐Lauraine E, Wagner U, Aavall‐Lundqvist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum‐sensitive ovarian cancer in late relapse. Journal of Clinical Oncology 2010;28(20):3323‐9. - PubMed
Campbell 1989
CTCAE 2006
-
- CTCAE. Common Terminology Criteria for Adverse Events v3.0 (CTCAE), 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/....
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining for heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Healthcare: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
EMA 2010
-
- European Medicines Agency. EPARs for authorised medicinal products for human use. http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for... 2010 (accessed 24 January 2013).
EMA 2011
-
- European Medicines Agency. Avastin (bevacizumab) product page. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Info....
EUROCARE‐4 2009
-
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE‐4. Survival of cancer patients diagnosed in 1995‐1999. Results and commentary. European Journal of Cancer 2009;45(6):931‐91. - PubMed
FIGO 2009
-
- FIGO CinGO. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. International Journal of Gynecology and Obstetrics 2009;105(1):3‐4. - PubMed
Gabizon 1997
-
- Gabizon A, Martin F. Polyethylene glycol‐coated (pegylated) liposomal doxorubicin: rationale for use in solid tumours. Drugs 1997;54(Suppl 4):15‐21. - PubMed
Gabizon 2001
-
- Gabizon AA. Stealth liposomes and tumour targeting: one step further in the quest for the magic bullet. Clinical Cancer Research 2001;7:223‐5. - PubMed
GLOBOCAN 2008
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. http://globocan.iarc.fr. Lyon, France.
HeCOG 2010
-
- Bafaloukos D, Linardou H, Aravantinos G, Papadimitriou G, Bamias A, Fountzilas G, et al. A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: a Hellenic Cooperative Oncology Group study. BMC Medicine 2010;8:3. - PMC - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jacobs 1988
-
- Jacobs I, Bridges J, Reynolds C, Stabile I, Kemsley P, Grudzinskas J, et al. Multimodal approach to screening for ovarian cancer. The Lancet 1988;331(8580):268‐71. - PubMed
Janssen‐Cilag 2011
-
- Janssen‐Cilag P, Ltd. CAELYX Product Information. http://www.janssen.com.au/Products/Caelyx. Amended 4 January 2011 (accessed 24 January 2013).
Katsumata 2009
-
- Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose‐dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open‐label, randomised controlled trial. The Lancet 2009;374(9698):1331‐8. - PubMed
Lawrie 2013
Markman 2010
-
- Markman M, Moon J, Wilczynski S, Lopez AM, Rowland KM, Michelin DP, et al. Single agent carboplatin versus carboplatin plus pegylated liposomal doxorubicin in recurrent ovarian cancer: final survival results of a SWOG (S0200) phase 3 randomized trial. Gynecologic Oncology 2010;116(3):323‐5. - PMC - PubMed
Menon 2009
-
- Menon U, Gentry‐Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). The Lancet Oncology 2009;10(4):327‐40. - PubMed
NICE 2003
-
- National Institute for Health and Clinical Excellence. Guidance on the use of paclitaxel in the treatment of ovarian cancer (TA55). London: National Institute for Health and Clinical Excellence, 2003.
NICE 2011
-
- National Institute for Health and Clinical Excellence. Ovarian cancer: the recognition and initial management of ovarian cancer (CG122). London: National Institute for Health and Clinical Excellence 2011.
OCMP 1991
-
- Ovarian Cancer Meta‐analysis Project. Cyclophosphamide plus cisplatin versus cyclophosphamide, doxorubicin and cisplatin chemotherapy of ovarian carcinoma. Journal of Clinical Oncology 1991;9:1668‐74. - PubMed
Ozols 1980
-
- Ozols RF, Wilson JK, Weltz MD, Grotzinger KR, Myers CE, Young RC. Inhibition of human ovarian cancer colony formation by adriamycin and its major metabolites. Cancer Research 1980;40:4109‐12. - PubMed
Perren 2011
-
- Perren TJ, Swart AM, Pfisterer J, Ledemann JA, Pujade‐Lauraine E, Kristensen G, and the ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. New England Journal of Medicine 2011;365:2484‐96. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
SEER 2007
-
- Kosary CL. Chapter 16: Cancer of the ovary. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M‐J editor(s). Cancer Survival Among Adults: US SEER Program, 1988‐2001. Bethesda: US Department of Health and Human Services; National Cancer Institute, 2007:133‐44.
Stark 2013
-
- Stark D, Nankivell M, Pujade‐Lauraine E, Kristensen G, Elit L, Stockler M, et al. Standard chemotherapy with or without bevacizumab in advanced ovarian cancer: quality‐of‐life outcomes from the International Collaboration on Ovarian Neoplasms (ICON7) phase 3 randomised trial. The Lancet Oncology 2013;14(3):236‐43. - PMC - PubMed
Therasse 2000
-
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205‐16. - PubMed
Waterhouse 2001
-
- Waterhouse DN, Tardi PG, Mayer LD, Bally MB. A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Safety 2001;24:903‐20. - PubMed
Zunino 2002
-
- Zunino F, Pratesi G. Antitumour antibodies. Oxford Textbook of Oncology. 2nd Edition. Oxford, UK: Oxford University Press, 2002:715‐27.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

