Use of expert panels to define the reference standard in diagnostic research: a systematic review of published methods and reporting
- PMID: 24143138
- PMCID: PMC3797139
- DOI: 10.1371/journal.pmed.1001531
Use of expert panels to define the reference standard in diagnostic research: a systematic review of published methods and reporting
Abstract
Background: In diagnostic studies, a single and error-free test that can be used as the reference (gold) standard often does not exist. One solution is the use of panel diagnosis, i.e., a group of experts who assess the results from multiple tests to reach a final diagnosis in each patient. Although panel diagnosis, also known as consensus or expert diagnosis, is frequently used as the reference standard, guidance on preferred methodology is lacking. The aim of this study is to provide an overview of methods used in panel diagnoses and to provide initial guidance on the use and reporting of panel diagnosis as reference standard.
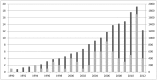
Methods and findings: PubMed was systematically searched for diagnostic studies applying a panel diagnosis as reference standard published up to May 31, 2012. We included diagnostic studies in which the final diagnosis was made by two or more persons based on results from multiple tests. General study characteristics and details of panel methodology were extracted. Eighty-one studies were included, of which most reported on psychiatry (37%) and cardiovascular (21%) diseases. Data extraction was hampered by incomplete reporting; one or more pieces of critical information about panel reference standard methodology was missing in 83% of studies. In most studies (75%), the panel consisted of three or fewer members. Panel members were blinded to the results of the index test results in 31% of studies. Reproducibility of the decision process was assessed in 17 (21%) studies. Reported details on panel constitution, information for diagnosis and methods of decision making varied considerably between studies.
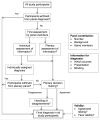
Conclusions: Methods of panel diagnosis varied substantially across studies and many aspects of the procedure were either unclear or not reported. On the basis of our review, we identified areas for improvement and developed a checklist and flow chart for initial guidance for researchers conducting and reporting of studies involving panel diagnosis. Please see later in the article for the Editors' Summary.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Diagnostic test accuracy and cost-effectiveness of tests for codeletion of chromosomal arms 1p and 19q in people with glioma.Cochrane Database Syst Rev. 2022 Mar 2;3(3):CD013387. doi: 10.1002/14651858.CD013387.pub2. Cochrane Database Syst Rev. 2022. PMID: 35233774 Free PMC article.
-
123I-MIBG scintigraphy and 18F-FDG-PET imaging for diagnosing neuroblastoma.Cochrane Database Syst Rev. 2015 Sep 29;2015(9):CD009263. doi: 10.1002/14651858.CD009263.pub2. Cochrane Database Syst Rev. 2015. PMID: 26417712 Free PMC article.
-
Regional cerebral blood flow single photon emission computed tomography for detection of Frontotemporal dementia in people with suspected dementia.Cochrane Database Syst Rev. 2015 Jun 23;2015(6):CD010896. doi: 10.1002/14651858.CD010896.pub2. Cochrane Database Syst Rev. 2015. PMID: 26102272 Free PMC article.
-
The educational effects of portfolios on undergraduate student learning: a Best Evidence Medical Education (BEME) systematic review. BEME Guide No. 11.Med Teach. 2009 Apr;31(4):282-98. doi: 10.1080/01421590902889897. Med Teach. 2009. PMID: 19404891
Cited by
-
The Sports Cardiology Team: Personalizing Athlete Care Through a Comprehensive, Multidisciplinary Approach.Mayo Clin Proc Innov Qual Outcomes. 2022 Oct 12;6(6):525-535. doi: 10.1016/j.mayocpiqo.2022.08.006. eCollection 2022 Dec. Mayo Clin Proc Innov Qual Outcomes. 2022. PMID: 36267910 Free PMC article.
-
People with dementia in nursing home research: a methodological review of the definition and identification of the study population.BMC Geriatr. 2016 Apr 5;16:78. doi: 10.1186/s12877-016-0249-7. BMC Geriatr. 2016. PMID: 27052960 Free PMC article. Review.
-
Candidate Biomarkers for the Diagnosis of Transient Ischemic Attack: A Systematic Review.Cerebrovasc Dis. 2019;47(5-6):207-216. doi: 10.1159/000502449. Epub 2019 Aug 30. Cerebrovasc Dis. 2019. PMID: 31473737 Free PMC article.
-
Epidemiology of heart failure.Eur J Heart Fail. 2020 Aug;22(8):1342-1356. doi: 10.1002/ejhf.1858. Epub 2020 Jun 1. Eur J Heart Fail. 2020. PMID: 32483830 Free PMC article. Review.
-
Comparative Test Evaluation: Methods and Challenges.J Gambl Stud. 2018 Dec;34(4):1109-1138. doi: 10.1007/s10899-018-9745-3. J Gambl Stud. 2018. PMID: 29368061
References
-
- Reitsma JB, Rutjes AW, Khan KS, Coomarasamy A, Bossuyt PM (2009) A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. J Clin Epidemiol 62: 797–806. - PubMed
-
- Hadgu A, Dendukuri N, Hilden J (2005) Evaluation of nucleic acid amplification tests in the absence of a perfect gold-standard test: a review of the statistical and epidemiologic issues. Epidemiology 16: 604–612. - PubMed
-
- Alonzo TA, Pepe MS (1999) Using a combination of reference tests to assess the accuracy of a new diagnostic test. Stat Med 18: 2987–3003. - PubMed
-
- Pepe MS, Janes H (2007) Insights into latent class analysis of diagnostic test performance. Biostatistics 8: 474–484. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

