Minnelide: a novel therapeutic that promotes apoptosis in non-small cell lung carcinoma in vivo
- PMID: 24143232
- PMCID: PMC3797124
- DOI: 10.1371/journal.pone.0077411
Minnelide: a novel therapeutic that promotes apoptosis in non-small cell lung carcinoma in vivo
Abstract
Background: Minnelide, a pro-drug of triptolide, has recently emerged as a potent anticancer agent. The precise mechanisms of its cytotoxic effects remain unclear.
Methods: Cell viability was studied using CCK8 assay. Cell proliferation was measured real-time on cultured cells using Electric Cell Substrate Impedence Sensing (ECIS). Apoptosis was assayed by Caspase activity on cultured lung cancer cells and TUNEL staining on tissue sections. Expression of pro-survival and anti-apoptotic genes (HSP70, BIRC5, BIRC4, BIRC2, UACA, APAF-1) was estimated by qRTPCR. Effect of Minnelide on proliferative cells in the tissue was estimated by Ki-67 staining of animal tissue sections.
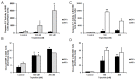
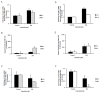
Results: In this study, we investigated in vitro and in vivo antitumor effects of triptolide/Minnelide in non-small cell lung carcinoma (NSCLC). Triptolide/Minnelide exhibited anti-proliferative effects and induced apoptosis in NSCLC cell lines and NSCLC mouse models. Triptolide/Minnelide significantly down-regulated the expression of pro-survival and anti-apoptotic genes (HSP70, BIRC5, BIRC4, BIRC2, UACA) and up-regulated pro-apoptotic APAF-1 gene, in part, via attenuating the NF-κB signaling activity.
Conclusion: In conclusion, our results provide supporting mechanistic evidence for Minnelide as a potential in NSCLC.
Conflict of interest statement
Figures




Similar articles
-
Downregulation of Sp1 by Minnelide leads to decrease in HSP70 and decrease in tumor burden of gastric cancer.PLoS One. 2017 Feb 13;12(2):e0171827. doi: 10.1371/journal.pone.0171827. eCollection 2017. PLoS One. 2017. PMID: 28192510 Free PMC article.
-
Inhibition of epithelial ovarian cancer by Minnelide, a water-soluble pro-drug.Gynecol Oncol. 2014 Nov;135(2):318-24. doi: 10.1016/j.ygyno.2014.08.031. Epub 2014 Aug 27. Gynecol Oncol. 2014. PMID: 25172764 Free PMC article.
-
Minnelide reduces tumor burden in preclinical models of osteosarcoma.Cancer Lett. 2013 Jul 28;335(2):412-20. doi: 10.1016/j.canlet.2013.02.050. Epub 2013 Mar 14. Cancer Lett. 2013. PMID: 23499892 Free PMC article.
-
[Advances on effects of triptolide with non-small cell lung cancer].Zhongguo Fei Ai Za Zhi. 2013 Jul;16(7):378-81. doi: 10.3779/j.issn.1009-3419.2013.07.09. Zhongguo Fei Ai Za Zhi. 2013. PMID: 23866670 Free PMC article. Review. Chinese.
-
"Heat shock protein 70 in pancreatic diseases: Friend or foe".J Surg Oncol. 2017 Jul;116(1):114-122. doi: 10.1002/jso.24653. Epub 2017 May 22. J Surg Oncol. 2017. PMID: 28543919 Free PMC article. Review.
Cited by
-
Reactive oxygen species-driven mitochondrial injury induces apoptosis by teroxirone in human non-small cell lung cancer cells.Oncol Lett. 2017 Sep;14(3):3503-3509. doi: 10.3892/ol.2017.6586. Epub 2017 Jul 15. Oncol Lett. 2017. PMID: 28927105 Free PMC article.
-
Minnelide/Triptolide Impairs Mitochondrial Function by Regulating SIRT3 in P53-Dependent Manner in Non-Small Cell Lung Cancer.PLoS One. 2016 Aug 8;11(8):e0160783. doi: 10.1371/journal.pone.0160783. eCollection 2016. PLoS One. 2016. PMID: 27501149 Free PMC article.
-
HSP70 Multi-Functionality in Cancer.Cells. 2020 Mar 2;9(3):587. doi: 10.3390/cells9030587. Cells. 2020. PMID: 32121660 Free PMC article. Review.
-
Development of Potential Antitumor Agents from the Scaffolds of Plant-Derived Terpenoid Lactones.J Med Chem. 2020 Dec 24;63(24):15410-15448. doi: 10.1021/acs.jmedchem.0c01449. Epub 2020 Dec 8. J Med Chem. 2020. PMID: 33289552 Free PMC article.
-
Triptolide induces apoptotic cell death of human cholangiocarcinoma cells through inhibition of myeloid cell leukemia-1.BMC Cancer. 2014 Apr 17;14:271. doi: 10.1186/1471-2407-14-271. BMC Cancer. 2014. PMID: 24742042 Free PMC article.
References
-
- American Cancer Society (2013). ancer Facts Figures 2013. Available: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume.... Accessed 13 March, 2013.
-
- Ries L, Eisner M, Kosary C et al. (2013) ancer Statistics Review, 1975-2002, Bethesda Md, NCI, 2005. Available: http://seer.cancer.gov/csr/1975_2007/index.html. Accessed 13 August 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

