Inhibition of human MCF-7 breast cancer cells and HT-29 colon cancer cells by rice-produced recombinant human insulin-like growth binding protein-3 (rhIGFBP-3)
- PMID: 24143239
- PMCID: PMC3797122
- DOI: 10.1371/journal.pone.0077516
Inhibition of human MCF-7 breast cancer cells and HT-29 colon cancer cells by rice-produced recombinant human insulin-like growth binding protein-3 (rhIGFBP-3)
Abstract
Background: Insulin-like growth factor binding protein-3 (IGFBP-3) is a multifunctional molecule which is closely related to cell growth, apoptosis, angiogenesis, metabolism and senescence. It combines with insulin-like growth factor-I (IGF-I) to form a complex (IGF-I/IGFBP-3) that can treat growth hormone insensitivity syndrome (GHIS) and reduce insulin requirement in patients with diabetes. IGFBP-3 alone has been shown to have anti-proliferation effect on numerous cancer cells.
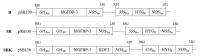
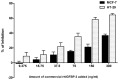
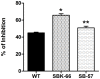
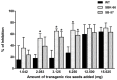
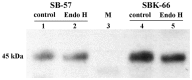
Methodology/principal findings: We reported here an expression method to produce functional recombinant human IGFBP-3 (rhIGFBP-3) in transgenic rice grains. Protein sorting sequences, signal peptide and endoplasmic reticulum retention tetrapeptide (KDEL) were included in constructs for enhancing rhIGFBP-3 expression. Western blot analysis showed that only the constructs with signal peptide were successfully expressed in transgenic rice grains. Both rhIGFBP-3 proteins, with or without KDEL sorting sequence inhibited the growth of MCF-7 human breast cancer cells (65.76 ± 1.72% vs 45.00 ± 0.86%, p < 0.05; 50.84 ± 1.97% vs 45.00 ± 0.86%, p < 0.01 respectively) and HT-29 colon cancer cells (65.14 ± 3.84% vs 18.01 ± 13.81%, p < 0.05 and 54.7 ± 9.44% vs 18.01 ± 13.81%, p < 0.05 respectively) when compared with wild type rice.
Conclusion/significance: These findings demonstrated the feasibility of producing biological active rhIGFBP-3 in rice using a transgenic approach, which will definitely encourage more research on the therapeutic use of hIGFBP-3 in future.
Conflict of interest statement
Figures


Similar articles
-
Insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) functions as an IGF-reversible inhibitor of IGFBP-4 proteolysis.J Biol Chem. 1995 Nov 17;270(46):27481-8. doi: 10.1074/jbc.270.46.27481. J Biol Chem. 1995. PMID: 7499205
-
Mecasermin rinfabate: insulin-like growth factor-I/insulin-like growth factor binding protein-3, mecaserimin rinfibate, rhIGF-I/rhIGFBP-3.Drugs R D. 2005;6(2):120-7. doi: 10.2165/00126839-200506020-00008. Drugs R D. 2005. PMID: 15777106 Review.
-
Expression and subcellular targeting of human insulin-like growth factor binding protein-3 in transgenic tobacco plants.Transgenic Res. 2009 Dec;18(6):943-51. doi: 10.1007/s11248-009-9286-8. Epub 2009 Jun 6. Transgenic Res. 2009. PMID: 19504171
-
Recombinant human insulin-like growth factor-binding protein 3 inhibits tumor growth and targets the Akt pathway in lung and colon cancer models.Growth Horm IGF Res. 2008 Dec;18(6):487-96. doi: 10.1016/j.ghir.2008.04.002. Epub 2008 May 23. Growth Horm IGF Res. 2008. PMID: 18502161
-
Antiproliferative actions of insulin-like growth factor binding protein (IGFBP)-3 in human breast cancer cells.Prog Growth Factor Res. 1995;6(2-4):503-12. doi: 10.1016/0955-2235(95)00025-9. Prog Growth Factor Res. 1995. PMID: 8817695 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

