Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the broomrape family
- PMID: 24143802
- PMCID: PMC3877813
- DOI: 10.1105/tpc.113.113373
Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the broomrape family
Abstract
Nonphotosynthetic plants possess strongly reconfigured plastomes attributable to convergent losses of photosynthesis and housekeeping genes, making them excellent systems for studying genome evolution under relaxed selective pressures. We report the complete plastomes of 10 photosynthetic and nonphotosynthetic parasites plus their nonparasitic sister from the broomrape family (Orobanchaceae). By reconstructing the history of gene losses and genome reconfigurations, we find that the establishment of obligate parasitism triggers the relaxation of selective constraints. Partly because of independent losses of one inverted repeat region, Orobanchaceae plastomes vary 3.5-fold in size, with 45 kb in American squawroot (Conopholis americana) representing the smallest plastome reported from land plants. Of the 42 to 74 retained unique genes, only 16 protein genes, 15 tRNAs, and four rRNAs are commonly found. Several holoparasites retain ATP synthase genes with intact open reading frames, suggesting a prolonged function in these plants. The loss of photosynthesis alters the chromosomal architecture in that recombinogenic factors accumulate, fostering large-scale chromosomal rearrangements as functional reduction proceeds. The retention of DNA fragments is strongly influenced by both their proximity to genes under selection and the co-occurrence with those in operons, indicating complex constraints beyond gene function that determine the evolutionary survival time of plastid regions in nonphotosynthetic plants.
Figures

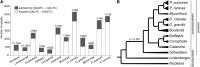



Comment in
-
Plastid genes that were lost along the road to parasitism.Plant Cell. 2013 Oct;25(10):3636. doi: 10.1105/tpc.113.251011. Epub 2013 Oct 18. Plant Cell. 2013. PMID: 24143804 Free PMC article. No abstract available.
References
-
- Bennett J.R., Mathews S. (2006). Phylogeny of the parasitic plant family Orobanchaceae inferred from phytochrome A. Am. J. Bot. 93: 1039–1051 - PubMed
-
- Berg S., Krause K., Krupinska K. (2004). The rbcL genes of two Cuscuta species, C. gronovii and C. subinclusa, are transcribed by the nuclear-encoded plastid RNA polymerase (NEP). Planta 219: 541–546 - PubMed
-
- Bock, R. (2007). Structure, function, and inheritance of plastid genomes. In Cell and Molecular Biology of Plastids, Topics in Current Genetics, R. Bock, ed (Berlin and Heidelberg: Springer), pp. 29–63.
-
- Braukmann T., Stefanović S. (2012). Plastid genome evolution in mycoheterotrophic Ericaceae. Plant Mol. Biol. 79: 5–20 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

