A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation
- PMID: 24143808
- PMCID: PMC3816427
- DOI: 10.1073/pnas.1316981110
A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation
Abstract
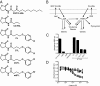
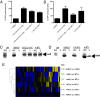
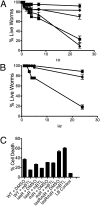
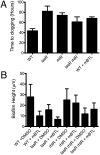
Quorum sensing is a chemical communication process that bacteria use to regulate collective behaviors. Disabling quorum-sensing circuits with small molecules has been proposed as a potential strategy to prevent bacterial pathogenicity. The human pathogen Pseudomonas aeruginosa uses quorum sensing to control virulence and biofilm formation. Here, we analyze synthetic molecules for inhibition of the two P. aeruginosa quorum-sensing receptors, LasR and RhlR. Our most effective compound, meta-bromo-thiolactone (mBTL), inhibits both the production of the virulence factor pyocyanin and biofilm formation. mBTL also protects Caenorhabditis elegans and human lung epithelial cells from killing by P. aeruginosa. Both LasR and RhlR are partially inhibited by mBTL in vivo and in vitro; however, RhlR, not LasR, is the relevant in vivo target. More potent antagonists do not exhibit superior function in impeding virulence. Because LasR and RhlR reciprocally control crucial virulence factors, appropriately tuning rather than completely inhibiting their activities appears to hold the key to blocking pathogenesis in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Comment in
-
Antimicrobials: silencing bacterial communication.Nat Rev Microbiol. 2013 Dec;11(12):820-1. doi: 10.1038/nrmicro3163. Epub 2013 Nov 6. Nat Rev Microbiol. 2013. PMID: 24192650 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

