HIF-1α activation results in actin cytoskeleton reorganization and modulation of Rac-1 signaling in endothelial cells
- PMID: 24144209
- PMCID: PMC3895861
- DOI: 10.1186/1478-811X-11-80
HIF-1α activation results in actin cytoskeleton reorganization and modulation of Rac-1 signaling in endothelial cells
Abstract
Background: Hypoxia is a major driving force in vascularization and vascular remodeling. Pharmacological inhibition of prolyl hydroxylases (PHDs) leads to an oxygen-independent and long-lasting activation of hypoxia-inducible factors (HIFs). Whereas effects of HIF-stabilization on transcriptional responses have been thoroughly investigated in endothelial cells, the molecular details of cytoskeletal changes elicited by PHD-inhibition remain largely unknown. To investigate this important aspect of PHD-inhibition, we used a spheroid-on-matrix cell culture model.
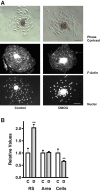
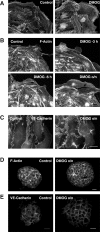
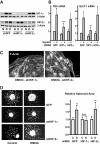
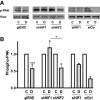
Results: Microvascular endothelial cells (glEND.2) were organized into spheroids. Migration of cells from the spheroids was quantified and analyzed by immunocytochemistry. The PHD inhibitor dimethyloxalyl glycine (DMOG) induced F-actin stress fiber formation in migrating cells, but only weakly affected microvascular endothelial cells firmly attached in a monolayer. Compared to control spheroids, the residual spheroids were larger upon PHD inhibition and contained more cells with tight VE-cadherin positive cell-cell contacts. Morphological alterations were dependent on stabilization of HIF-1α and not HIF-2α as shown in cells with stable knockdown of HIF-α isoforms. DMOG-treated endothelial cells exhibited a reduction of immunoreactive Rac-1 at the migrating front, concomitant with a diminished Rac-1 activity, whereas total Rac-1 protein remained unchanged. Two chemically distinct Rac-1 inhibitors mimicked the effects of DMOG in terms of F-actin fiber formation and orientation, as well as stabilization of residual spheroids. Furthermore, phosphorylation of p21-activated kinase PAK downstream of Rac-1 was reduced by DMOG in a HIF-1α-dependent manner. Stabilization of cell-cell contacts associated with decreased Rac-1 activity was also confirmed in human umbilical vein endothelial cells.
Conclusions: Our data demonstrates that PHD inhibition induces HIF-1α-dependent cytoskeletal remodeling in endothelial cells, which is mediated essentially by a reduction in Rac-1 signaling.
Figures



Similar articles
-
Sestrin2 inhibits hypoxia-inducible factor-1α accumulation via AMPK-mediated prolyl hydroxylase regulation.Free Radic Biol Med. 2016 Dec;101:511-523. doi: 10.1016/j.freeradbiomed.2016.11.014. Epub 2016 Nov 11. Free Radic Biol Med. 2016. PMID: 27840318
-
Increased activation of the hypoxia-inducible factor pathway in varicose veins.J Vasc Surg. 2012 May;55(5):1427-39. doi: 10.1016/j.jvs.2011.10.111. Epub 2012 Jan 24. J Vasc Surg. 2012. PMID: 22277691
-
Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung.Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10212-7. doi: 10.1073/pnas.0504520102. Epub 2005 Jul 11. Proc Natl Acad Sci U S A. 2005. PMID: 16009933 Free PMC article.
-
Protein Hydroxylation by Hypoxia-Inducible Factor (HIF) Hydroxylases: Unique or Ubiquitous?Cells. 2019 Apr 26;8(5):384. doi: 10.3390/cells8050384. Cells. 2019. PMID: 31035491 Free PMC article. Review.
-
Prolyl Hydroxylase Inhibitors: a New Opportunity in Renal and Myocardial Protection.Cardiovasc Drugs Ther. 2022 Dec;36(6):1187-1196. doi: 10.1007/s10557-021-07257-0. Epub 2021 Sep 17. Cardiovasc Drugs Ther. 2022. PMID: 34533692 Review.
Cited by
-
Dephosphorylation of Y685-VE-Cadherin Involved in Pulmonary Microvascular Endothelial Barrier Injury Induced by Angiotensin II.Mediators Inflamm. 2016;2016:8696481. doi: 10.1155/2016/8696481. Epub 2016 Dec 29. Mediators Inflamm. 2016. PMID: 28119542 Free PMC article.
-
Nanoscale Systems for Local Activation of Hypoxia-Inducible Factor-1 Alpha: A New Approach in Diabetic Wound Management.Int J Nanomedicine. 2024 Dec 21;19:13735-13762. doi: 10.2147/IJN.S497041. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39723173 Free PMC article. Review.
-
High-Resolution Secretome Analysis of Chemical Hypoxia Treated Cells Identifies Putative Biomarkers of Chondrosarcoma.Proteomes. 2022 Jul 20;10(3):25. doi: 10.3390/proteomes10030025. Proteomes. 2022. PMID: 35893766 Free PMC article.
-
Copper depletion inhibits CoCl2-induced aggressive phenotype of MCF-7 cells via downregulation of HIF-1 and inhibition of Snail/Twist-mediated epithelial-mesenchymal transition.Sci Rep. 2015 Jul 15;5:12410. doi: 10.1038/srep12410. Sci Rep. 2015. PMID: 26174737 Free PMC article.
-
A regulatory microRNA network controls endothelial cell phenotypic switch during sprouting angiogenesis.Elife. 2020 Jan 24;9:e48095. doi: 10.7554/eLife.48095. Elife. 2020. PMID: 31976858 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

