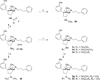
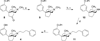4β-Methyl-5-(3-hydroxyphenyl)morphan opioid agonist and partial agonist derived from a 4β-methyl-5-(3-hydroxyphenyl)morphan pure antagonist
- PMID: 24144404
- PMCID: PMC3893112
- DOI: 10.1021/jm401250s
4β-Methyl-5-(3-hydroxyphenyl)morphan opioid agonist and partial agonist derived from a 4β-methyl-5-(3-hydroxyphenyl)morphan pure antagonist
Abstract
In previous studies we reported that addition of 7α-acylamino groups to N-phenylpropyl-4β-methyl-5-(3-hydroxyphenyl)morphan (4) led to compounds that were pure opioid receptor antagonists. In contrast to these findings we report in this study that addition of a 7α-amino (5a), 7α-alkylamino (5b-e), or 7α-dialkylamino (5f-h) group to 4 leads to opioid receptor ligands with varying degrees of agonist/antagonist activity. The 7α-amino and 7α-methylamino analogues were full agonists at the μ and δ receptors and antagonists at the κ receptor. The 7α-cyclopropylmethylamino analogue 5h was a full agonist at the μ receptor with weaker agonist activity at the δ and κ receptors. Whereas the addition of a 7α-acylamino group to the pure nonselective opioid receptor antagonist N-phenylpropyl-4β-methyl-5-(3-hydroxyphenyl)morphan (4) led to κ selective pure opioid receptor antagonist, the addition of a 7α-amino, 7α-alkylamino, or 7α-dialkylamino group to 4 leads to opioid ligands that are largely μ or δ agonist with mixed agonist/antagonist properties.
Figures
Similar articles
-
N-substituted 4beta-methyl-5-(3-hydroxyphenyl)-7alpha-amidomorphans are potent, selective kappa opioid receptor antagonists.J Med Chem. 2006 Mar 9;49(5):1781-91. doi: 10.1021/jm058264p. J Med Chem. 2006. PMID: 16509593
-
Probes for narcotic receptor mediated phenomena. 44. Synthesis of an N-substituted 4-hydroxy-5-(3-hydroxyphenyl)morphan with high affinity and selective μ-antagonist activity.Eur J Med Chem. 2012 Apr;50:44-54. doi: 10.1016/j.ejmech.2012.01.025. Epub 2012 Jan 20. Eur J Med Chem. 2012. PMID: 22341895 Free PMC article.
-
Discovery of an opioid kappa receptor selective pure antagonist from a library of N-substituted 4beta-methyl-5-(3-hydroxyphenyl)morphans.J Med Chem. 2002 Aug 1;45(16):3524-30. doi: 10.1021/jm020084h. J Med Chem. 2002. PMID: 12139463
-
Rational drug design of selective epsilon opioid receptor agonist TAN-821 and antagonist TAN-1014.Curr Med Chem. 2006;13(10):1109-18. doi: 10.2174/092986706776360851. Curr Med Chem. 2006. PMID: 16719773 Review.
-
The TIPP opioid peptide family: development of delta antagonists, delta agonists, and mixed mu agonist/delta antagonists.Biopolymers. 1999;51(6):411-25. doi: 10.1002/(SICI)1097-0282(1999)51:6<411::AID-BIP4>3.0.CO;2-Z. Biopolymers. 1999. PMID: 10797230 Review.
Cited by
-
Unified Enantioselective Synthesis of 5-Phenylmorphans and cis-Octahydroisoquinolines.Org Lett. 2025 Aug 15;27(32):8941-8945. doi: 10.1021/acs.orglett.5c02600. Epub 2025 Aug 1. Org Lett. 2025. PMID: 40748297 Free PMC article.
-
Diastereoselective Synthesis of Morphan Derivatives by Michael and Hetero-Michael Addition of 1,1-Enediamines to Quinone Monoketals.ACS Omega. 2018 Jan 2;3(1):8-21. doi: 10.1021/acsomega.7b01493. eCollection 2018 Jan 31. ACS Omega. 2018. PMID: 31457875 Free PMC article.
-
μ Opioid receptor: novel antagonists and structural modeling.Sci Rep. 2016 Feb 18;6:21548. doi: 10.1038/srep21548. Sci Rep. 2016. PMID: 26888328 Free PMC article.
References
-
- Zimmerman DM, Nickander R, Horng JS, Wong DT. New structural concepts for narcotic antagonists defined in a 4-phenylpiperidine series. Nature. 1978;275:332–334. - PubMed
-
- Mitch CH, Leander JD, Mendelsohn LG, Shaw WN, Wong DT, Cantrell BE, Johnson BG, Reel JK, Snoddy JD, Takemori AE, Zimmerman DM. 3,4-Dimethyl-4-(3-hydroxyphenyl)piperidines: Opioid antagonists with potent anorectant activity. J. Med. Chem. 1993;36:2842–2850. - PubMed
-
- Thomas JB, Mascarella SW, Rothman RB, Partilla JS, Xu H, McCullough KB, Dersch CM, Cantrell BE, Zimmerman DM, Carroll FI. Investigation of the N-substituent conformation governing potency and mu receptor subtype-selectivity in (+)-(3R, 4R)-dimethyl-4-(3-hydroxyphenyl)piperidine opioid antagonists. J. Med. Chem. 1998;41:1980–1990. - PubMed
-
- Zimmerman DM, Gidda JS, Cantrell BE, Schoepp DD, Johnson BG, Leander JD. Discovery of a potent, peripherally selective trans-3,4-dimethyl-4-(3-hydroxyphenyl)piperidine opioid antagonist for the treatment of gastrointestinal motility disorders. J. Med. Chem. 1994;37:2262–2265. - PubMed
-
- Zimmerman DM, Leander JD, Cantrell BE, Reel JK, Snoddy J, Mendelsohn LG, Johnson BG, Mitch CH. Structure-activity relationships of the trans-3,4-dimethyl-4-(3-hydroxyphenyl)piperidine antagonists for μ and κ opioid receptors. J. Med. Chem. 1993;36:2833–2841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials