Recombinant Ehrlichia P29 protein induces a protective immune response in a mouse model of ehrlichiosis
- PMID: 24144475
- PMCID: PMC3893061
- DOI: 10.1016/j.vaccine.2013.10.036
Recombinant Ehrlichia P29 protein induces a protective immune response in a mouse model of ehrlichiosis
Abstract
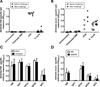
Ehrlichioses are emerging tick-borne bacterial diseases of humans and animals for which no vaccines are available. The diseases are caused by obligately intracellular bacteria belonging to the genus Ehrlichia. Several immunoreactive proteins of ehrlichiae have been identified based on their reactivity with immune sera from human patients and animals. These include the major outer membrane proteins, ankyrin repeat proteins and tandem repeat proteins (TRP). Polyclonal antibodies directed against the tandem repeats (TRs) of Ehrlichia chaffeensis TRP32, TRP47 and TRP120 have been shown to provide protection in mice. In the present study, we evaluated E. muris P29, which is the ortholog of E. chaffeensis TRP47 and E. canis TRP36, as a subunit vaccine in a mouse model of ehrlichiosis. Our study indicated that unlike E. chaffeensis TRP47 and E. canis TRP36, orthologs of E. muris (P29) and E. muris-like agent (EMLA) do not contain tandem repeats. Immunization of mice with recombinant E. muris P29 induced significant protection against a challenge infection. The protection induced by E. muris P29 was associated with induction of strong antibody responses. In contrast to development of P29-specific IgG antibodies following immunization, development of P29-specific IgG antibodies, but not IgM antibodies, was impaired during persistent E. muris infection. Furthermore, our study indicated that CD4+ T cells target P29 during E. muris infection and differentiate into IFN-γ-producing Th1 effector/memory cells. In conclusion, our study indicated that orthologs of E. muris P29 showed considerable variation in the central tandem repeat region among different species, induction of P29-specific IgG antibody response was impaired during persistent E. muris infection, and rP29 induced protective immune responses.
Keywords: Antibody; Antigen; Ehrlichia; Intracellular bacteria; Protective immunity; Vaccine.
Published by Elsevier Ltd.
Conflict of interest statement
The authors have no financial conflicts of interest
Figures


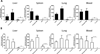

Similar articles
-
Immunization with Ehrlichia P28 outer membrane proteins confers protection in a mouse model of ehrlichiosis.Clin Vaccine Immunol. 2011 Dec;18(12):2018-25. doi: 10.1128/CVI.05292-11. Epub 2011 Oct 26. Clin Vaccine Immunol. 2011. PMID: 22030371 Free PMC article.
-
CD4 T-cell epitopes associated with protective immunity induced following vaccination of mice with an ehrlichial variable outer membrane protein.Infect Immun. 2007 Nov;75(11):5453-9. doi: 10.1128/IAI.00713-07. Epub 2007 Aug 13. Infect Immun. 2007. PMID: 17698576 Free PMC article.
-
Protective heterologous immunity against fatal ehrlichiosis and lack of protection following homologous challenge.Infect Immun. 2008 May;76(5):1920-30. doi: 10.1128/IAI.01293-07. Epub 2008 Feb 19. Infect Immun. 2008. PMID: 18285501 Free PMC article.
-
Ehrlichioses: An Important One Health Opportunity.Vet Sci. 2016 Aug 31;3(3):20. doi: 10.3390/vetsci3030020. Vet Sci. 2016. PMID: 29056728 Free PMC article. Review.
-
Ehrlichia chaffeensis: a prevalent, life-threatening, emerging pathogen.Trans Am Clin Climatol Assoc. 2004;115:375-82; discussion 382-4. Trans Am Clin Climatol Assoc. 2004. PMID: 17060980 Free PMC article. Review.
Cited by
-
Effect of GP19 Peptide Hyperimmune Antiserum on Activated Macrophage during Ehrlichia canis Infection in Canine Macrophage-like Cells.Animals (Basel). 2021 Aug 5;11(8):2310. doi: 10.3390/ani11082310. Animals (Basel). 2021. PMID: 34438767 Free PMC article.
-
Recombinant Ehrlichia canis GP19 Protein as a Promising Vaccine Prototype Providing a Protective Immune Response in a Mouse Model.Vet Sci. 2022 Jul 27;9(8):386. doi: 10.3390/vetsci9080386. Vet Sci. 2022. PMID: 36006302 Free PMC article.
-
Immune Response to Tick-Borne Hemoparasites: Host Adaptive Immune Response Mechanisms as Potential Targets for Therapies and Vaccines.Int J Mol Sci. 2020 Nov 20;21(22):8813. doi: 10.3390/ijms21228813. Int J Mol Sci. 2020. PMID: 33233869 Free PMC article. Review.
-
Attenuated Mutants of Ehrlichia chaffeensis Induce Protection against Wild-Type Infection Challenge in the Reservoir Host and in an Incidental Host.Infect Immun. 2015 Jul;83(7):2827-35. doi: 10.1128/IAI.00487-15. Epub 2015 Apr 27. Infect Immun. 2015. PMID: 25916990 Free PMC article.
-
Vaccine development: obligate intracellular bacteria new tools, old pathogens: the current state of vaccines against obligate intracellular bacteria.Front Cell Infect Microbiol. 2024 Mar 19;14:1282183. doi: 10.3389/fcimb.2024.1282183. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38567021 Free PMC article. Review.
References
-
- Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, et al. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341(3):148–155. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

