Stilbene 5c, a microtubule poison with vascular disrupting properties that induces multiple modes of growth arrest and cell death
- PMID: 24144631
- PMCID: PMC3855433
- DOI: 10.1016/j.bcp.2013.10.007
Stilbene 5c, a microtubule poison with vascular disrupting properties that induces multiple modes of growth arrest and cell death
Abstract
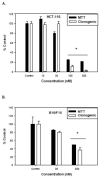
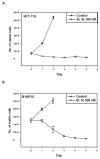
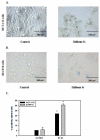
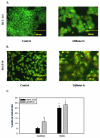
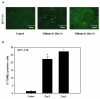
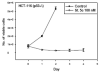
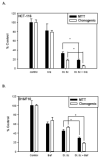
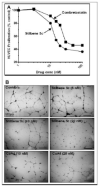
The stilbene derivative, cis-3,4',5-trimethoxy-3'-aminostilbene (stilbene 5c), is a potentially potent antitumor agent that acts via binding to the colchicine-binding site in tubulin. The current studies were designed to investigate the effectiveness of stilbene 5c against the HCT-116 human colon cancer cell line and B16/F10 melanoma cells as well as human endothelial cell tube formation and tumor perfusion. Stilbene 5c produced a time-dependent decrease in cell viability in both cell lines and the capacity of the cells to proliferate was not restored upon removal of the drug. Treatment with stilbene 5c also promoted both senescence and autophagy in both cell lines. TUNEL and annexin 5 staining indicated that apoptosis also occurs in stilbene 5c-treated HCT-116 cells, but not in B16/F10 melanoma cells. DAPI staining revealed morphological changes in the cell nuclei (binucleated and micronucleated cells) indicative of mitotic catastrophe in HCT-116 cells but not in the B16/F10 melanoma cells. p53-null HCT-116 cells demonstrated a similar growth arrest/cell death response to stilbene as p53-wild type HCT-116 cells. Stilbene 5c also completely inhibited human endothelial cell tube formation on Matrigel, consistent with potential anti-angiogenic actions. Using a new method developed for monitoring the pharmacodynamic effects of stilbene 5c in vivo, we found that a single injection of stilbene 5c reduced tumor perfusion by 65% at 4h, returning to baseline by 24h, while subsequent daily injections of stilbene 5c produced progressively larger reductions and smaller rebounds. This work indicates that stilbene 5c could potentially be effective against melanoma and colon cancer through the promotion of multiple modes of growth arrest and cell death coupled with anti-angiogenic and antivascular actions.
Keywords: Angiogenic; Autophagy; Microtubules; Senescence; Vascular disrupting.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Development of water soluble derivatives of cis-3, 4', 5-trimethoxy-3'-aminostilbene for optimization and use in cancer therapy.Invest New Drugs. 2009 Feb;27(1):41-52. doi: 10.1007/s10637-008-9139-y. Epub 2008 May 31. Invest New Drugs. 2009. PMID: 18516499
-
Autophagy, cell death and sustained senescence arrest in B16/F10 melanoma cells and HCT-116 colon carcinoma cells in response to the novel microtubule poison, JG-03-14.Cancer Chemother Pharmacol. 2013 Feb;71(2):441-55. doi: 10.1007/s00280-012-2024-6. Epub 2012 Nov 21. Cancer Chemother Pharmacol. 2013. PMID: 23178952
-
cis-3, 4', 5-Trimethoxy-3'-aminostilbene disrupts tumor vascular perfusion without damaging normal organ perfusion.Cancer Chemother Pharmacol. 2009 Jan;63(2):191-200. doi: 10.1007/s00280-008-0726-6. Epub 2008 Mar 26. Cancer Chemother Pharmacol. 2009. PMID: 18365199
-
Tubulin-interactive stilbene derivatives as anticancer agents.Cell Mol Biol Lett. 2013 Sep;18(3):368-97. doi: 10.2478/s11658-013-0094-z. Epub 2013 Jul 1. Cell Mol Biol Lett. 2013. PMID: 23818224 Free PMC article. Review.
-
If not apoptosis, then what? Treatment-induced senescence and mitotic catastrophe in tumor cells.Drug Resist Updat. 2001 Oct;4(5):303-13. doi: 10.1054/drup.2001.0213. Drug Resist Updat. 2001. PMID: 11991684 Review.
Cited by
-
Non-Canonical Programmed Cell Death in Colon Cancer.Cancers (Basel). 2022 Jul 7;14(14):3309. doi: 10.3390/cancers14143309. Cancers (Basel). 2022. PMID: 35884370 Free PMC article. Review.
-
Colchicine induces autophagy and senescence in lung cancer cells at clinically admissible concentration: potential use of colchicine in combination with autophagy inhibitor in cancer therapy.Tumour Biol. 2016 Aug;37(8):10653-64. doi: 10.1007/s13277-016-4972-7. Epub 2016 Feb 11. Tumour Biol. 2016. PMID: 26867767
-
Dynamic bioluminescence and fluorescence imaging of the effects of the antivascular agent Combretastatin-A4P (CA4P) on brain tumor xenografts.Cancer Lett. 2015 Jan 28;356(2 Pt B):462-9. doi: 10.1016/j.canlet.2014.09.038. Epub 2014 Oct 8. Cancer Lett. 2015. PMID: 25305449 Free PMC article.
References
-
- Ohtsu A. Chemotherapy for metastatic gastric cancer: Past, present, and future. J Gastroenterol. 2008;43:256–64. - PubMed
-
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. - PubMed
-
- Eigentler TK, Caroli UM, Radny P, Garbe C. Palliative therapy of disseminated malignant melanoma: A systematic review of 41 randomised clinical trials. Lancet Oncol. 2003;4:748–59. - PubMed
-
- Avril MF, Aamdal S, Grob JJ, Hauschild A, Mohr P, Bonerandi JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: A phase iii study. J Clin Oncol. 2004;22:1118–25. - PubMed
-
- Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: The oblimersen melanoma study group. J Clin Oncol. 2006;24:4738–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

