Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes
- PMID: 24144653
- PMCID: PMC3836105
- DOI: 10.2337/dc13-0234
Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes
Erratum in
- Diabetes Care. 2014 Feb;37(2):587
Abstract
Objective: To assess the efficacy and safety of 32 mg naltrexone sustained-release (SR)/360 mg bupropion SR (NB) in overweight/obese individuals with type 2 diabetes with or without background oral antidiabetes drugs.
Research design and methods: This was a 56-week, double-blind, placebo-controlled study in which 505 patients received standardized lifestyle intervention and were randomized 2:1 to NB or placebo. Coprimary end points were percent weight change and achievement of ≥5% weight loss. Secondary end points included achievement of HbA1c <7% (53 mmol/mol), achievement of weight loss ≥10%, and change in HbA1c, waist circumference, fasting blood glucose, and lipids.
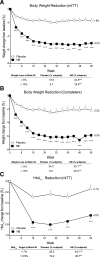
Results: In the modified intent-to-treat population (54% female, 80% Caucasian, and mean age 54 years, weight 106 kg, BMI 37 kg/m(2), and HbA1c 8.0% [64 mmol/mol]), NB resulted in significantly greater weight reduction (-5.0 vs. -1.8%; P < 0.001) and proportion of patients achieving ≥5% weight loss (44.5 vs. 18.9%, P < 0.001) compared with placebo. NB also resulted in significantly greater HbA1c reduction (-0.6 vs. -0.1% [6.6 vs. 1.1 mmol/mol]; P < 0.001), percent of patients achieving HbA1c <7% (53 mmol/mol) (44.1 vs. 26.3%; P < 0.001), and improvement in triglycerides and HDL cholesterol compared with placebo. NB was associated with higher incidence of nausea (42.3 vs. 7.1%), constipation (17.7 vs. 7.1%), and vomiting (18.3 vs. 3.6%). No difference was observed between groups in the incidence of depression, suicidal ideation, or hypoglycemia.
Conclusions: NB therapy in overweight/obese patients with type 2 diabetes induced weight loss, which was associated with improvements in glycemic control and select cardiovascular risk factors and was generally well tolerated with a safety profile similar to that in patients without diabetes.
Figures
References
-
- Bays HE, González-Campoy JM, Bray GA, et al. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 2008;6:343–368 - PubMed
-
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–497 - PubMed
-
- Centers for Disease Control and Prevention (CDC) Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988-1994 and 1999-2002. MMWR Morb Mortal Wkly Rep 2004;53:1066–1068 - PubMed
-
- Niswender K. Diabetes and obesity: therapeutic targeting and risk reduction - a complex interplay. Diabetes Obes Metab 2010;12:267–287 - PubMed
-
- Hollander PA, Kushner P. Type 2 diabetes comorbidities and treatment challenges: rationale for DPP-4 inhibitors. Postgrad Med 2010;122:71–80 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

