Using registries to identify adverse events in rheumatic diseases
- PMID: 24144710
- PMCID: PMC3813393
- DOI: 10.1542/peds.2013-0755
Using registries to identify adverse events in rheumatic diseases
Abstract
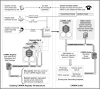
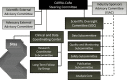
The proven effectiveness of biologics and other immunomodulatory products in inflammatory rheumatic diseases has resulted in their widespread use as well as reports of potential short- and long-term complications such as infection and malignancy. These complications are especially worrisome in children who often have serial exposures to multiple immunomodulatory products. Post-marketing surveillance of immunomodulatory products in juvenile idiopathic arthritis (JIA) and pediatric systemic lupus erythematosus is currently based on product-specific registries and passive surveillance, which may not accurately reflect the safety risks for children owing to low numbers, poor long-term retention, and inadequate comparators. In collaboration with the US Food and Drug Administration (FDA), patient and family advocacy groups, biopharmaceutical industry representatives and other stakeholders, the Childhood Arthritis and Rheumatology Research Alliance (CARRA) and the Duke Clinical Research Institute (DCRI) have developed a novel pharmacosurveillance model (CARRA Consolidated Safety Registry [CoRe]) based on a multicenter longitudinal pediatric rheumatic diseases registry with over 8000 participants. The existing CARRA infrastructure provides access to much larger numbers of subjects than is feasible in single-product registries. Enrollment regardless of medication exposure allows more accurate detection and evaluation of safety signals. Flexibility built into the model allows the addition of specific data elements and safety outcomes, and designation of appropriate disease comparator groups relevant to each product, fulfilling post-marketing requirements and commitments. The proposed model can be applied to other pediatric and adult diseases, potentially transforming the paradigm of pharmacosurveillance in response to the growing public mandate for rigorous post-marketing safety monitoring.
Keywords: biologic products; immunomodulatory therapy; juvenile rheumatic disease; medication safety.
Figures

References
-
- Lovell DJ, Giannini EH, Reiff A, et al. Pediatric Rheumatology Collaborative Study Group . Etanercept in children with polyarticular juvenile rheumatoid arthritis. N Engl J Med. 2000;342(11):763–769 - PubMed
-
- Weinblatt ME, Kremer JM, Bankhurst AD, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340(4):253–259 - PubMed
-
- Rodriguez W, Selen A, Avant D, et al. Improving pediatric dosing through pediatric initiatives: what we have learned. Pediatrics. 2008;121(3):530–539 - PubMed
-
- Diak P, Siegel J, La Grenade L, Choi L, Lemery S, McMahon A. Tumor necrosis factor alpha blockers and malignancy in children: forty-eight cases reported to the Food and Drug Administration. Arthritis Rheum. 2010;62(8):2517–2524 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

