Effect of mature blood-stage Plasmodium parasite sequestration on pathogen biomass in mathematical and in vivo models of malaria
- PMID: 24144725
- PMCID: PMC3911827
- DOI: 10.1128/IAI.00705-13
Effect of mature blood-stage Plasmodium parasite sequestration on pathogen biomass in mathematical and in vivo models of malaria
Abstract
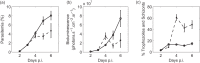
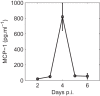
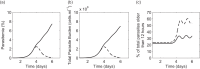
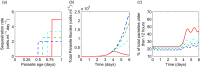
Parasite biomass and microvasculature obstruction are strongly associated with disease severity and death in Plasmodium falciparum-infected humans. This is related to sequestration of mature, blood-stage parasites (schizonts) in peripheral tissue. The prevailing view is that schizont sequestration leads to an increase in pathogen biomass, yet direct experimental data to support this are lacking. Here, we first studied parasite population dynamics in inbred wild-type (WT) mice infected with the rodent species of malaria, Plasmodium berghei ANKA. As is commonly reported, these mice became moribund due to large numbers of parasites in multiple tissues. We then studied infection dynamics in a genetically targeted line of mice, which displayed minimal tissue accumulation of parasites. We constructed a mathematical model of parasite biomass dynamics, incorporating schizont-specific host clearance, both with and without schizont sequestration. Combined use of mathematical and in vivo modeling indicated, first, that the slowing of parasite growth in the genetically targeted mice can be attributed to specific clearance of schizonts from the circulation and, second, that persistent parasite growth in WT mice can be explained solely as a result of schizont sequestration. Our work provides evidence that schizont sequestration could be a major biological process driving rapid, early increases in parasite biomass during blood-stage Plasmodium infection.
Figures


Similar articles
-
Bioluminescence imaging of P. berghei Schizont sequestration in rodents.Methods Mol Biol. 2013;923:353-68. doi: 10.1007/978-1-62703-026-7_25. Methods Mol Biol. 2013. PMID: 22990791
-
Reduced CD36-dependent tissue sequestration of Plasmodium-infected erythrocytes is detrimental to malaria parasite growth in vivo.J Exp Med. 2012 Jan 16;209(1):93-107. doi: 10.1084/jem.20110762. Epub 2011 Dec 19. J Exp Med. 2012. PMID: 22184632 Free PMC article.
-
Sequestration and tissue accumulation of human malaria parasites: can we learn anything from rodent models of malaria?PLoS Pathog. 2010 Sep 30;6(9):e1001032. doi: 10.1371/journal.ppat.1001032. PLoS Pathog. 2010. PMID: 20941396 Free PMC article. Review.
-
ICAM-1 is a key receptor mediating cytoadherence and pathology in the Plasmodium chabaudi malaria model.Malar J. 2017 May 3;16(1):185. doi: 10.1186/s12936-017-1834-8. Malar J. 2017. PMID: 28468674 Free PMC article.
-
Host-mediated impairment of parasite maturation during blood-stage Plasmodium infection.Proc Natl Acad Sci U S A. 2017 Jul 18;114(29):7701-7706. doi: 10.1073/pnas.1618939114. Epub 2017 Jul 3. Proc Natl Acad Sci U S A. 2017. PMID: 28673996 Free PMC article.
Cited by
-
Systemic host inflammation induces stage-specific transcriptomic modification and slower maturation in malaria parasites.mBio. 2023 Aug 31;14(4):e0112923. doi: 10.1128/mbio.01129-23. Epub 2023 Jul 14. mBio. 2023. PMID: 37449844 Free PMC article.
-
Parasite Viability as a Measure of In Vivo Drug Activity in Preclinical and Early Clinical Antimalarial Drug Assessment.Antimicrob Agents Chemother. 2022 Jul 19;66(7):e0011422. doi: 10.1128/aac.00114-22. Epub 2022 Jun 21. Antimicrob Agents Chemother. 2022. PMID: 35727057 Free PMC article.
-
Pathogenetic mechanisms and treatment targets in cerebral malaria.Nat Rev Neurol. 2023 Nov;19(11):688-709. doi: 10.1038/s41582-023-00881-4. Epub 2023 Oct 19. Nat Rev Neurol. 2023. PMID: 37857843 Review.
-
Effects of lemon decoction on malaria parasite clearance and selected hematological parameters in Plasmodium berghei ANKA infected mice.BMC Complement Med Ther. 2020 Jan 30;20(1):24. doi: 10.1186/s12906-020-2820-1. BMC Complement Med Ther. 2020. PMID: 32020885 Free PMC article.
-
Infection length and host environment influence on Plasmodium falciparum dry season reservoir.EMBO Mol Med. 2024 Oct;16(10):2349-2375. doi: 10.1038/s44321-024-00127-w. Epub 2024 Sep 16. EMBO Mol Med. 2024. PMID: 39284949 Free PMC article.
References
-
- World Health Organization 2011. World malaria report 2011 (WHO Global Malaria Programme), 13th ed. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/world_malaria_report_2011/9789241564403_eng.pdf
-
- World Health Organization 2000. Severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 94:S1–S90 - PubMed
-
- Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, Newton PN, Pitisuttithum P, Smithyman AM, White NJ, Day NPJ. 2005. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2:e204. 10.1371/journal.pmed.0020204 - DOI - PMC - PubMed
-
- Hanson J, Lam SWK, Mahanta KC, Pattnaik R, Alam S, Mohanty S, Hasan MU, Hossain A, Charunwatthana P, Chotivanich K, Maude RJ, Kingston H, Day NP, Mishra S, White NJ, Dondorp AM. 2012. Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J. Infect. Dis. 206:571–579. 10.1093/infdis/jis400 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

