The developmental stages of synaptic plasticity
- PMID: 24144877
- PMCID: PMC3903349
- DOI: 10.1113/jphysiol.2012.235119
The developmental stages of synaptic plasticity
Abstract
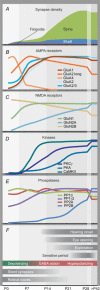
The brain is programmed to drive behaviour by precisely wiring the appropriate neuronal circuits. Wiring and rewiring of neuronal circuits largely depends on the orchestrated changes in the strengths of synaptic contacts. Here, we review how the rules of synaptic plasticity change during development of the brain, from birth to independence. We focus on the changes that occur at the postsynaptic side of excitatory glutamatergic synapses in the rodent hippocampus and neocortex. First we summarize the current data on the structure of synapses and the developmental expression patterns of the key molecular players of synaptic plasticity, N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, as well as pivotal kinases (Ca(2+)/calmodulin-dependent protein kinase II, protein kinase A, protein kinase C) and phosphatases (PP1, PP2A, PP2B). In the second part we relate these findings to important characteristics of the emerging network. We argue that the concerted and gradual shifts in the usage of plasticity molecules comply with the changing need for (re)wiring neuronal circuits.
Figures
Similar articles
-
Rules of engagement: factors that regulate activity-dependent synaptic plasticity during neural network development.Biol Bull. 2010 Oct;219(2):81-99. doi: 10.1086/BBLv219n2p81. Biol Bull. 2010. PMID: 20972254 Review.
-
Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors.Eur J Neurosci. 2006 Apr;23(8):2035-47. doi: 10.1111/j.1460-9568.2006.04733.x. Eur J Neurosci. 2006. PMID: 16630051
-
Defective synaptic transmission and structure in the dentate gyrus and selective fear memory impairment in the Rsk2 mutant mouse model of Coffin-Lowry syndrome.Neurobiol Dis. 2013 Oct;58:156-68. doi: 10.1016/j.nbd.2013.05.016. Epub 2013 Jun 3. Neurobiol Dis. 2013. PMID: 23742761
-
Regulation of neuronal PKA signaling through AKAP targeting dynamics.Eur J Cell Biol. 2006 Jul;85(7):627-33. doi: 10.1016/j.ejcb.2006.01.010. Epub 2006 Feb 28. Eur J Cell Biol. 2006. PMID: 16504338 Review.
-
Transiently higher release probability during critical period at thalamocortical synapses in the mouse barrel cortex: relevance to differential short-term plasticity of AMPA and NMDA EPSCs and possible involvement of silent synapses.Eur J Neurosci. 2004 Dec;20(11):3006-18. doi: 10.1111/j.1460-9568.2004.03756.x. Eur J Neurosci. 2004. PMID: 15579155
Cited by
-
Activity-regulated E3 ubiquitin ligase TRIM47 modulates excitatory synapse development.Front Mol Neurosci. 2022 Sep 21;15:943980. doi: 10.3389/fnmol.2022.943980. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36211980 Free PMC article.
-
Unraveling why we sleep: Quantitative analysis reveals abrupt transition from neural reorganization to repair in early development.Sci Adv. 2020 Sep 18;6(38):eaba0398. doi: 10.1126/sciadv.aba0398. Print 2020 Sep. Sci Adv. 2020. PMID: 32948580 Free PMC article.
-
Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer's disease animal model.J Neuroinflammation. 2019 Dec 13;16(1):264. doi: 10.1186/s12974-019-1665-3. J Neuroinflammation. 2019. PMID: 31836020 Free PMC article.
-
Alterations in the Sp1 binding and Fmr-1 gene expression in the cortex of the brain during maturation and aging of mouse.Mol Biol Rep. 2014 Oct;41(10):6855-63. doi: 10.1007/s11033-014-3571-1. Epub 2014 Jul 12. Mol Biol Rep. 2014. PMID: 25015265
-
Effects of Patterned Sound Deprivation on Short- and Long-Term Plasticity in the Rat Thalamocortical Auditory System In Vivo.Neural Plast. 2016;2016:3407135. doi: 10.1155/2016/3407135. Epub 2016 Jan 4. Neural Plast. 2016. PMID: 26881106 Free PMC article.
References
-
- Akaneya Y( Activity regulates the expression of AMPA receptor subunit GluR4 in developing visual cortex. Eur J Neurosci. 2007;25:1641–1646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

