Site nurse-initiated adherence and symptom support telephone calls for HIV-positive individuals starting antiretroviral therapy, ACTG 5031: substudy of ACTG 384
- PMID: 24144900
- PMCID: PMC4019013
- DOI: 10.1310/hct1405-235
Site nurse-initiated adherence and symptom support telephone calls for HIV-positive individuals starting antiretroviral therapy, ACTG 5031: substudy of ACTG 384
Abstract
Background: Effective and easy to implement interventions to improve adherence to antiretroviral therapy are needed.
Objective: To compare site nurse-initiated adherence and symptom support telephone calls for HIV-positive individuals starting antiretroviral therapy to the study site's standard of care.
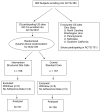
Methods: A randomized controlled trial of site nurse-initiated adherence and symptom support telephone calls for HIV-positive individuals starting antiretroviral therapy. Subjects were randomized to receive site nurse-initiated telephone calls (intervention) or no additional calls to the site's standard of care (control). Subjects received calls 1 to 3 days after initiating antiretrovirals, on weeks 1, 2, 3, 6, 10, 14, 18, 22, and 26, and every 8 weeks thereafter. Self-reported adherence was captured during study visits.
Results: A total of 333 subjects starting antiretrovirals as part of ACTG 384 were co-enrolled into ACTG 5031. Subjects were followed for up to 160 weeks and were contacted for 74% of scheduled calls. There was no significant difference in proportion of patients with ≯95% mean total adherence (87.9% and 91.2%; P = .34) and mean self-reported total adherence (97.9% and 98.4%) in the intervention and control groups, respectively, or in symptom distress and clinical endpoints.
Conclusions: In the context of a clinical trial where self-reported adherence was exceptionally high, the site nurse-initiated telephone calls did not further improve self-reported adherence, symptom distress, or clinical outcomes.
Keywords: adherence intervention; antiretroviral therapy; human immunodeficiency virus (HIV); nursing telephone support; randomized controlled trial.
Figures


References
-
- Walensky RP, Paltiel AD, Losina E, et al. The survival benefits of AIDS treatment in the united states. J Infect Dis. 2006;194(1):11–19. - PubMed
-
- Chesney MA, Ickovics J, Hecht FM, Sikipa G, Rabkin J. Adherence: A necessity for successful HIV combination therapy. AIDS. 1999;13(Suppl A):S271–8. - PubMed
-
- Reynolds NR. Adherence to antiretroviral therapies: State of the science. Curr HIV Res. 2004;2(3):207–214. - PubMed
-
- Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. - PubMed
-
- Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clin Infect Dis. 2006;43(7):939–941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI069511/AI/NIAID NIH HHS/United States
- K01 AI062435/AI/NIAID NIH HHS/United States
- AI069501/AI/NIAID NIH HHS/United States
- AI069452/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- AI069470/AI/NIAID NIH HHS/United States
- AI069424/AI/NIAID NIH HHS/United States
- AI062435/AI/NIAID NIH HHS/United States
- AI069450/AI/NIAID NIH HHS/United States
- U01 AI027659/AI/NIAID NIH HHS/United States
- UM1AI068636/AI/NIAID NIH HHS/United States
- AI069415/AI/NIAID NIH HHS/United States
- AI069556/AI/NIAID NIH HHS/United States
- AI069465/AI/NIAID NIH HHS/United States
- AI069532/AI/NIAID NIH HHS/United States
- AI069434/AI/NIAID NIH HHS/United States
- AI069513/AI/NIAID NIH HHS/United States
- AI069502/AI/NIAID NIH HHS/United States
- AI069428/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- AI069472/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- AI069432/AI/NIAID NIH HHS/United States
- AI069477/AI/NIAID NIH HHS/United States
- AI069471/AI/NIAID NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
