Microtubule dynamic instability controls podosome patterning in osteoclasts through EB1, cortactin, and Src
- PMID: 24144981
- PMCID: PMC3911273
- DOI: 10.1128/MCB.00578-13
Microtubule dynamic instability controls podosome patterning in osteoclasts through EB1, cortactin, and Src
Abstract
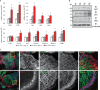
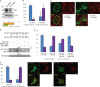
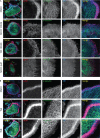
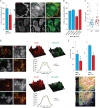
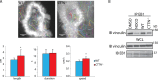
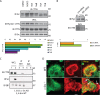
In osteoclasts (OCs) podosomes are organized in a belt, a feature critical for bone resorption. Although microtubules (MTs) promote the formation and stability of the belt, the MT and/or podosome molecules that mediate the interaction of the two systems are not identified. Because the growing "plus" ends of MTs point toward the podosome belt, plus-end tracking proteins (+TIPs) might regulate podosome patterning. Among the +TIPs, EB1 increased as OCs matured and was enriched in the podosome belt, and EB1-positive MTs targeted podosomes. Suppression of MT dynamic instability, displacement of EB1 from MT ends, or EB1 depletion resulted in the loss of the podosome belt. We identified cortactin as an Src-dependent interacting partner of EB1. Cortactin-deficient OCs presented a defective MT targeting to, and patterning of, podosomes and reduced bone resorption. Suppression of MT dynamic instability or EB1 depletion increased cortactin phosphorylation, decreasing its acetylation and affecting its interaction with EB1. Thus, dynamic MTs and podosomes interact to control bone resorption.
Figures

References
-
- Baron R, Neff L, Brown W, Courtoy PJ, Louvard D, Farquhar MG. 1988. Polarized secretion of lysosomal enzymes: co-distribution of cation-independent mannose-6-phosphate receptors and lysosomal enzymes along the osteoclast exocytic pathway. J. Cell Biol. 106:1863–1872. 10.1083/jcb.106.6.1863 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
