TRPV1 antagonist capsazepine suppresses 4-AP-induced epileptiform activity in vitro and electrographic seizures in vivo
- PMID: 24145133
- PMCID: PMC4104534
- DOI: 10.1016/j.expneurol.2013.10.010
TRPV1 antagonist capsazepine suppresses 4-AP-induced epileptiform activity in vitro and electrographic seizures in vivo
Abstract
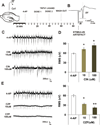
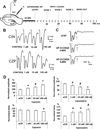
Transient receptor potential vanilloid 1 (TRPV1) is a cation-permeable ion channel found in the peripheral and central nervous systems. The membrane surface expression of TRPV1 is known to occur in neuronal cell bodies and sensory neuron axons. TRPV1 receptors are also expressed in the hippocampus, the main epileptogenic region in the brain. Although, previous studies implicate TRPV1 channels in the generation of epilepsy, suppression of ongoing seizures by TRPV1 antagonists has not yet been attempted. Here, we evaluate the role of TRPV1 channels in the modulation of epileptiform activity as well as the anti-convulsant properties of capsazepine (CZP), an established TRPV1 competitive antagonist, using in vitro and in vivo models. To this end, we used 4-aminopyridine (4-AP) to trigger seizure-like activity. We found that CZP suppressed 4-AP induced epileptiform activity in vitro (10-100μM) and in vivo (50mg/kg s.c.). In contrast, capsaicin enhanced 4-AP induced epileptiform activity in vitro (1-100μM) and triggered bursting activity in vivo (100μM dialysis perfusion), which was abolished by the TRPV1 antagonist CZP. To further investigate the mechanisms of TRPV1 modulation, we studied the effect of capsaicin and CZP on evoked potentials. Capsaicin (1-100μM) and CZP (10-100μM) increased and decreased, respectively, the amplitude of extracellular field evoked potentials in a concentration-dependent manner. Additional in vitro studies showed that the effect of the TRPV1 blocker on evoked potentials was similar whether the response was orthodromic or antidromic, suggesting that the effect involves interference with membrane depolarization on cell bodies and axons. The fact that CZP could act directly on axons was confirmed by decreased amplitude of the compound action potential and by an increased delay of both the antidromic potentials and the axonal response. Histological studies using transgenic mice also show that, in addition to the known neural expression, TRPV1 channels are widely expressed in alvear oligodendrocytes in the hippocampus. Taken together, these results indicate that activation of TRPV1 channels leads to enhanced excitability, while their inhibition can effectively suppress ongoing electrographic seizures. These results support a role for TRPV1 channels in the suppression of convulsive activity, indicating that antagonism of TRPV1 channels particularly in axons may possibly be a novel target for effective acute suppression of seizures.
Keywords: 4-AP; Capsaicin; Capsazepine; Electrophysiology; Epilepsy; Oligodendrocytes; TRPV1 channels.
© 2013.
Figures







References
-
- Afrah AW, Stiller CO, Olgart L, Brodin E, Gustafsson H. Involvement of spinal N-methyl-Daspartate receptors in capsaicin-induced in vivo release of substance P in the rat dorsal horn. Neurosci Lett. 2001;316:83–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

