Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements
- PMID: 24145165
- PMCID: PMC3812964
- DOI: 10.1083/jcb.201304063
Microtubules that form the stationary lattice of muscle fibers are dynamic and nucleated at Golgi elements
Abstract
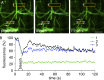
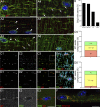
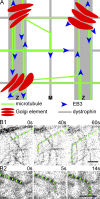
Skeletal muscle microtubules (MTs) form a nonclassic grid-like network, which has so far been documented in static images only. We have now observed and analyzed dynamics of GFP constructs of MT and Golgi markers in single live fibers and in the whole mouse muscle in vivo. Using confocal, intravital, and superresolution microscopy, we find that muscle MTs are dynamic, growing at the typical speed of ∼9 µm/min, and forming small bundles that build a durable network. We also show that static Golgi elements, associated with the MT-organizing center proteins γ-tubulin and pericentrin, are major sites of muscle MT nucleation, in addition to the previously identified sites (i.e., nuclear membranes). These data give us a framework for understanding how muscle MTs organize and how they contribute to the pathology of muscle diseases such as Duchenne muscular dystrophy.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

