Doxorubicin in TAT peptide-modified multifunctional immunoliposomes demonstrates increased activity against both drug-sensitive and drug-resistant ovarian cancer models
- PMID: 24145298
- PMCID: PMC3938525
- DOI: 10.4161/cbt.26609
Doxorubicin in TAT peptide-modified multifunctional immunoliposomes demonstrates increased activity against both drug-sensitive and drug-resistant ovarian cancer models
Abstract
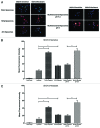
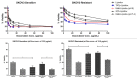
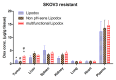
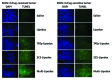
Multidrug resistance (MDR) is a hallmark of cancer cells and a crucial factor in chemotherapy failure, cancer reappearance, and patient deterioration. We have previously described the physicochemical characteristics and the in vitro anticancer properties of a multifunctional doxorubicin-loaded liposomal formulation. Lipodox(®), a commercially available PEGylated liposomal doxorubicin, was made multifunctional by surface-decorating with a cell-penetrating peptide, TATp, conjugated to PEG 1000-PE, to enhance liposomal cell uptake. A pH-sensitive polymer, PEG 2000-Hz-PE, with a pH-sensitive hydrazone (Hz) bond to shield the peptide in the body and expose it only at the acidic tumor cell surface, was used as well. In addition, an anti-nucleosome monoclonal antibody 2C5 attached to a long-chain polymer to target nucleosomes overexpressed on the tumor cell surface was also present. Here, we report the in vitro cell uptake and cytotoxicity of the modified multifunctional immunoliposomes as well as the in vivo studies on tumor xenografts developed subcutaneously in nude mice with MDR and drug-sensitive human ovarian cancer cells (SKOV-3). Our results show the ability of multifunctional immunoliposomes to overcome MDR by enhancing cytotoxicity in drug-resistant cells, compared with non-modified liposomes. Furthermore, in comparison with the non-modified liposomes, upon intravenous injection of these multifunctional immunoliposomes into mice with tumor xenografts, a significant reduction in tumor growth and enhanced therapeutic efficacy of the drug in both drug-resistant and drug-sensitive mice was obtained. The use of "smart" multifunctional delivery systems may provide the basis for an effective strategy to develop, improve, and overcome MDR cancers in the future.
Keywords: 2C5 mAb; Lipodox; SKOV-3 cells; TAT peptide; doxil; doxorubicin; multidrug resistance; multifunctional immunoliposomes; pH-sensitive polymers.
Figures

References
-
- Shaked Y, Emmenegger U, Francia G, Chen L, Lee CR, Man S, Paraghamian A, Ben-David Y, Kerbel RS. Low-dose metronomic combined with intermittent bolus-dose cyclophosphamide is an effective long-term chemotherapy treatment strategy. Cancer Res. 2005;65:7045–51. doi: 10.1158/0008-5472.CAN-05-0765. - DOI - PubMed
-
- Gottesman MM, Pastan I. Mechanisms of Drug-Resistance in Cancer-Therapy. Eur J Pharmacol. 1990;183:17–8. doi: 10.1016/0014-2999(90)91262-A. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
