Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma
- PMID: 24145345
- PMCID: PMC3837092
- DOI: 10.1200/JCO.2013.51.4802
Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma
Abstract
Purpose: Nivolumab, a human immunoglobulin G4-blocking antibody against the T-cell programmed death-1 checkpoint protein, has activity against metastatic melanoma. Its safety, clinical efficacy, and correlative biomarkers were assessed with or without a peptide vaccine in ipilimumab-refractory and -naive melanoma.
Patients and methods: In this phase I study, 90 patients with unresectable stage III or IV melanoma who were ipilimumab naive and had experienced progression after at least one prior therapy (cohorts 1 to 3, 34 patients) or experienced progression after prior ipilimumab (cohorts 4 to 6, 56 patients) received nivolumab at 1, 3, or 10 mg/kg every 2 weeks for 24 weeks, then every 12 weeks for up to 2 years, with or without a multipeptide vaccine.
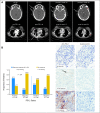
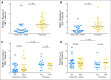
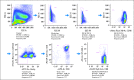
Results: Nivolumab with vaccine was well tolerated and safe at all doses. The RECIST 1.1 response rate for both ipilimumab-refractory and -naive patients was 25%. Median duration of response was not reached at a median of 8.1 months of follow-up. High pretreatment NY-ESO-1 and MART-1-specific CD8(+) T cells were associated with progression of disease. At week 12, increased peripheral-blood T regulatory cells and decreased antigen-specific T cells were associated with progression. PD-L1 tumor staining was associated with responses to nivolumab, but negative staining did not rule out a response. Patients who experienced progression after nivolumab could respond to ipilimumab.
Conclusion: In patients with ipilimumab-refractory or -naive melanoma, nivolumab at 3 mg/kg with or without peptide vaccine was well tolerated and induced responses lasting up to 140 weeks. Responses to nivolumab in ipilimumab-refractory patients or to ipilimumab in nivolumab-refractory patients support combination or sequencing of nivolumab and ipilimumab.
Trial registration: ClinicalTrials.gov NCT01176461.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

