PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer
- PMID: 24145346
- PMCID: PMC4881367
- DOI: 10.1200/JCO.2012.47.9626
PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer
Abstract
Purpose: PointBreak (A Study of Pemetrexed, Carboplatin and Bevacizumab in Patients With Nonsquamous Non-Small Cell Lung Cancer) compared the efficacy and safety of pemetrexed (Pem) plus carboplatin (C) plus bevacizumab (Bev) followed by pemetrexed plus bevacizumab (PemCBev) with paclitaxel (Pac) plus carboplatin (C) plus bevacizumab (Bev) followed by bevacizumab (PacCBev) in patients with advanced nonsquamous non-small-cell lung cancer (NSCLC).
Patients and methods: Patients with previously untreated stage IIIB or IV nonsquamous NSCLC and Eastern Cooperative Oncology Group performance status of 0 to 1 were randomly assigned to receive pemetrexed 500 mg/m(2) or paclitaxel 200 mg/m(2) combined with carboplatin area under the curve 6 and bevacizumab 15 mg/kg every 3 weeks for up to four cycles. Eligible patients received maintenance until disease progression: pemetrexed plus bevacizumab (for the PemCBev group) or bevacizumab (for the PacCBev group). The primary end point of this superiority study was overall survival (OS).
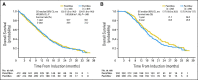
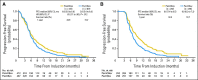
Results: Patients were randomly assigned to PemCBev (n = 472) or PacCBev (n = 467). For PemCBev versus PacCBev, OS hazard ratio (HR) was 1.00 (median OS, 12.6 v 13.4 months; P = .949); progression-free survival (PFS) HR was 0.83 (median PFS, 6.0 v 5.6 months; P = .012); overall response rate was 34.1% versus 33.0%; and disease control rate was 65.9% versus 69.8%. Significantly more study drug-related grade 3 or 4 anemia (14.5% v 2.7%), thrombocytopenia (23.3% v 5.6%), and fatigue (10.9% v 5.0%) occurred with PemCBev; significantly more grade 3 or 4 neutropenia (40.6% v 25.8%), febrile neutropenia (4.1% v 1.4%), sensory neuropathy (4.1% v 0%), and alopecia (grade 1 or 2; 36.8% v 6.6%) occurred with PacCBev.
Conclusion: OS did not improve with the PemCBev regimen compared with the PacCBev regimen, although PFS was significantly improved with PemCBev. Toxicity profiles differed; both regimens demonstrated tolerability.
Trial registration: ClinicalTrials.gov NCT00762034.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures



References
-
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. - PubMed
-
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. - PubMed
-
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer, Version 3, 2012. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
-
- D'Addario G, Früh M, Reck M, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21:v116–v119. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

