Tenascin-C is an innate broad-spectrum, HIV-1-neutralizing protein in breast milk
- PMID: 24145401
- PMCID: PMC3831436
- DOI: 10.1073/pnas.1307336110
Tenascin-C is an innate broad-spectrum, HIV-1-neutralizing protein in breast milk
Abstract
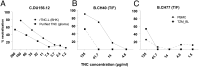
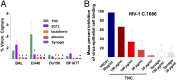
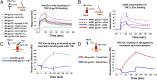
Achieving an AIDS-free generation will require elimination of postnatal transmission of HIV-1 while maintaining the nutritional and immunologic benefits of breastfeeding for infants in developing regions. Maternal/infant antiretroviral prophylaxis can reduce postnatal HIV-1 transmission, yet toxicities and the development of drug-resistant viral strains may limit the effectiveness of this strategy. Interestingly, in the absence of antiretroviral prophylaxis, greater than 90% of infants exposed to HIV-1 via breastfeeding remain uninfected, despite daily mucosal exposure to the virus for up to 2 y. Moreover, milk of uninfected women inherently neutralizes HIV-1 and prevents virus transmission in animal models, yet the factor(s) responsible for this anti-HIV activity is not well-defined. In this report, we identify a primary HIV-1-neutralizing protein in breast milk, Tenascin-C (TNC). TNC is an extracellular matrix protein important in fetal development and wound healing, yet its antimicrobial properties have not previously been established. Purified TNC captured and neutralized multiclade chronic and transmitted/founder HIV-1 variants, and depletion of TNC abolished the HIV-1-neutralizing activity of milk. TNC bound the HIV-1 Envelope protein at a site that is induced upon engagement of its primary receptor, CD4, and is blocked by V3 loop- (19B and F39F) and chemokine coreceptor binding site-directed (17B) monoclonal antibodies. Our results demonstrate the ability of an innate mucosal host protein found in milk to neutralize HIV-1 via binding to the chemokine coreceptor site, potentially explaining why the majority of HIV-1-exposed breastfed infants are protected against mucosal HIV-1 transmission.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The Presence and Anti-HIV-1 Function of Tenascin C in Breast Milk and Genital Fluids.PLoS One. 2016 May 16;11(5):e0155261. doi: 10.1371/journal.pone.0155261. eCollection 2016. PLoS One. 2016. PMID: 27182834 Free PMC article.
-
Breast Milk of HIV-Positive Mothers Has Potent and Species-Specific In Vivo HIV-Inhibitory Activity.J Virol. 2015 Nov;89(21):10868-78. doi: 10.1128/JVI.01702-15. Epub 2015 Aug 19. J Virol. 2015. PMID: 26292320 Free PMC article.
-
Use of antiretroviral drugs to prevent HIV-1 transmission through breast-feeding: from animal studies to randomized clinical trials.J Acquir Immune Defic Syndr. 2004 Feb 1;35(2):178-87. doi: 10.1097/00126334-200402010-00013. J Acquir Immune Defic Syndr. 2004. PMID: 14722452 Review.
-
Maternal but Not Infant Anti-HIV-1 Neutralizing Antibody Response Associates with Enhanced Transmission and Infant Morbidity.mBio. 2017 Oct 24;8(5):e01373-17. doi: 10.1128/mBio.01373-17. mBio. 2017. PMID: 29066544 Free PMC article.
-
Breast milk transmission of HIV-1. Laboratory and clinical studies.Ann N Y Acad Sci. 2000 Nov;918:122-7. doi: 10.1111/j.1749-6632.2000.tb05480.x. Ann N Y Acad Sci. 2000. PMID: 11131695 Review.
Cited by
-
Did Tenascin-C Co-Evolve With the General Immune System of Vertebrates?Front Immunol. 2021 Apr 12;12:663902. doi: 10.3389/fimmu.2021.663902. eCollection 2021. Front Immunol. 2021. PMID: 33912190 Free PMC article. Review.
-
A complex interplay between the extracellular matrix and the innate immune response to microbial pathogens.Immunology. 2018 Oct;155(2):186-201. doi: 10.1111/imm.12972. Epub 2018 Jul 5. Immunology. 2018. PMID: 29908065 Free PMC article. Review.
-
Role of intestinal extracellular matrix-related signaling in porcine epidemic diarrhea virus infection.Virulence. 2021 Dec;12(1):2352-2365. doi: 10.1080/21505594.2021.1972202. Virulence. 2021. PMID: 34515624 Free PMC article.
-
Effect of innate antiviral glycoproteins in breast milk on seroconversion to rotavirus vaccine (Rotarix) in children in Lusaka, Zambia.PLoS One. 2017 Dec 28;12(12):e0189351. doi: 10.1371/journal.pone.0189351. eCollection 2017. PLoS One. 2017. PMID: 29284036 Free PMC article.
-
The Human Milk Oligosaccharide Lacto-N-Fucopentaose III Conjugated to Dextran Inhibits HIV Replication in Primary Human Macrophages.Nutrients. 2025 Mar 2;17(5):890. doi: 10.3390/nu17050890. Nutrients. 2025. PMID: 40077760 Free PMC article.
References
-
- Kumwenda NI, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359(2):119–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

