Natural killer cell licensing in mice with inducible expression of MHC class I
- PMID: 24145414
- PMCID: PMC3831452
- DOI: 10.1073/pnas.1318255110
Natural killer cell licensing in mice with inducible expression of MHC class I
Abstract
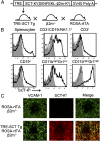
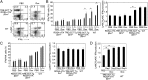
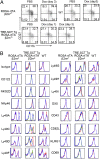
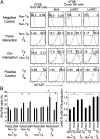
Mouse natural killer (NK) cells acquire effector function by an education process termed "licensing" mediated by inhibitory Ly49 receptors which recognize self-MHC class I. Ly49 receptors can bind to MHC class I on targets (in trans) and also to MHC class I on the NK-cell surface (in cis). Which of these interactions regulates NK-cell licensing is not yet clear. Moreover, there are no clear phenotypic differences between licensed and unlicensed NK cells, perhaps because of the previously limited ability to study NK cells with synchronized licensing. Here, we produced MHC class I-deficient mice with inducible MHC class I consisting of a single-chain trimer (SCT), ovalbumin peptide-β2 microgloblin-H2K(b) (SCT-K(b)). Only NK cells with a Ly49 receptor with specificity for SCT-K(b) were licensed after MHC class I induction. NK cells were localized consistently in red pulp of the spleen during induced NK-cell licensing, and there were no differences in maturation or activation markers on recently licensed NK cells. Although MHC class I-deficient NK cells were licensed in hosts following SCT-K(b) induction, NK cells were not licensed after induced SCT-K(b) expression on NK cells themselves in MHC class I-deficient hosts. Furthermore, hematopoietic cells with induced SCT-K(b) licensed NK cells more efficiently than stromal cells. These data indicate that trans interaction with MHC class I on hematopoietic cells regulates NK-cell licensing, which is not associated with other obvious phenotypic changes.
Keywords: immunity; lymphocytes; tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment.J Exp Med. 2010 Sep 27;207(10):2073-9. doi: 10.1084/jem.20100986. Epub 2010 Sep 6. J Exp Med. 2010. PMID: 20819924 Free PMC article.
-
Ly49-dependent NK cell licensing and effector inhibition involve the same interaction site on MHC ligands.J Immunol. 2011 Apr 1;186(7):3911-7. doi: 10.4049/jimmunol.1004168. Epub 2011 Feb 18. J Immunol. 2011. PMID: 21335486 Free PMC article.
-
Natural killer cell licensing during viral infection.Adv Exp Med Biol. 2011;780:37-44. doi: 10.1007/978-1-4419-5632-3_4. Adv Exp Med Biol. 2011. PMID: 21842363 Review.
-
Recognition of the nonclassical MHC class I molecule H2-M3 by the receptor Ly49A regulates the licensing and activation of NK cells.Nat Immunol. 2012 Dec;13(12):1171-7. doi: 10.1038/ni.2468. Epub 2012 Nov 11. Nat Immunol. 2012. PMID: 23142773 Free PMC article.
-
Selection, tuning, and adaptation in mouse NK cell education.Immunol Rev. 2015 Sep;267(1):167-77. doi: 10.1111/imr.12330. Immunol Rev. 2015. PMID: 26284477 Review.
Cited by
-
Cognate HLA absence in trans diminishes human NK cell education.J Clin Invest. 2016 Oct 3;126(10):3772-3782. doi: 10.1172/JCI86923. Epub 2016 Aug 29. J Clin Invest. 2016. PMID: 27571408 Free PMC article.
-
Differential Role of Hematopoietic and Nonhematopoietic Cell Types in the Regulation of NK Cell Tolerance and Responsiveness.J Immunol. 2016 Nov 15;197(10):4127-4136. doi: 10.4049/jimmunol.1402447. Epub 2016 Oct 19. J Immunol. 2016. PMID: 27798146 Free PMC article.
-
Adaptations of Natural Killer Cells to Self-MHC Class I.Front Immunol. 2014 Jul 22;5:349. doi: 10.3389/fimmu.2014.00349. eCollection 2014. Front Immunol. 2014. PMID: 25101089 Free PMC article.
-
High mTOR activity is a hallmark of reactive natural killer cells and amplifies early signaling through activating receptors.Elife. 2017 Sep 6;6:e26423. doi: 10.7554/eLife.26423. Elife. 2017. PMID: 28875936 Free PMC article.
-
Prenatal Allospecific NK Cell Tolerance Hinges on Instructive Allorecognition through the Activating Receptor during Development.J Immunol. 2015 Aug 15;195(4):1506-16. doi: 10.4049/jimmunol.1500463. Epub 2015 Jul 1. J Immunol. 2015. PMID: 26136432 Free PMC article.
References
-
- Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. - PubMed
-
- Yokoyama WM. Natural killer cells. In: Paul WE, editor. Fundamental Immunology. 7th Ed. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 395–431.
-
- Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358(6381):66–70. - PubMed
-
- Ljunggren HG, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today. 1990;11(7):237–244. - PubMed
-
- Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: Susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253(5016):199–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

