Plasmodium falciparum phosphoethanolamine methyltransferase is essential for malaria transmission
- PMID: 24145416
- PMCID: PMC3831454
- DOI: 10.1073/pnas.1313965110
Plasmodium falciparum phosphoethanolamine methyltransferase is essential for malaria transmission
Abstract
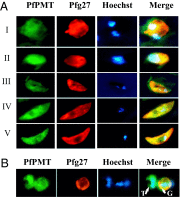
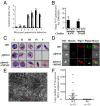
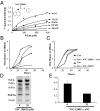
Efficient transmission of Plasmodium species between humans and Anopheles mosquitoes is a major contributor to the global burden of malaria. Gametocytogenesis, the process by which parasites switch from asexual replication within human erythrocytes to produce male and female gametocytes, is a critical step in malaria transmission and Plasmodium genetic diversity. Nothing is known about the pathways that regulate gametocytogenesis and only few of the current drugs that inhibit asexual replication are also capable of inhibiting gametocyte development and blocking malaria transmission. Here we provide genetic and pharmacological evidence indicating that the pathway for synthesis of phosphatidylcholine in Plasmodium falciparum membranes from host serine is essential for parasite gametocytogenesis and malaria transmission. Parasites lacking the phosphoethanolamine N-methyltransferase enzyme, which catalyzes the limiting step in this pathway, are severely altered in gametocyte development, are incapable of producing mature-stage gametocytes, and are not transmitted to mosquitoes. Chemical screening identified 11 inhibitors of phosphoethanolamine N-methyltransferase that block parasite intraerythrocytic asexual replication and gametocyte differentiation in the low micromolar range. Kinetic studies in vitro as well as functional complementation assays and lipid metabolic analyses in vivo on the most promising inhibitor NSC-158011 further demonstrated the specificity of inhibition. These studies set the stage for further optimization of NSC-158011 for development of a class of dual activity antimalarials to block both intraerythrocytic asexual replication and gametocytogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Alano P. Plasmodium falciparum gametocytes: Still many secrets of a hidden life. Mol Microbiol. 2007;66(2):291–302. - PubMed
-
- Pessi G, Choi JY, Reynolds JM, Voelker DR, Mamoun CB. In vivo evidence for the specificity of Plasmodium falciparum phosphoethanolamine methyltransferase and its coupling to the Kennedy pathway. J Biol Chem. 2005;280(13):12461–12466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

