Genome duplication and mutations in ACE2 cause multicellular, fast-sedimenting phenotypes in evolved Saccharomyces cerevisiae
- PMID: 24145419
- PMCID: PMC3831460
- DOI: 10.1073/pnas.1305949110
Genome duplication and mutations in ACE2 cause multicellular, fast-sedimenting phenotypes in evolved Saccharomyces cerevisiae
Abstract
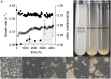
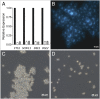
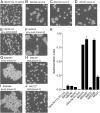
Laboratory evolution of the yeast Saccharomyces cerevisiae in bioreactor batch cultures yielded variants that grow as multicellular, fast-sedimenting clusters. Knowledge of the molecular basis of this phenomenon may contribute to the understanding of natural evolution of multicellularity and to manipulating cell sedimentation in laboratory and industrial applications of S. cerevisiae. Multicellular, fast-sedimenting lineages obtained from a haploid S. cerevisiae strain in two independent evolution experiments were analyzed by whole genome resequencing. The two evolved cell lines showed different frameshift mutations in a stretch of eight adenosines in ACE2, which encodes a transcriptional regulator involved in cell cycle control and mother-daughter cell separation. Introduction of the two ace2 mutant alleles into the haploid parental strain led to slow-sedimenting cell clusters that consisted of just a few cells, thus representing only a partial reconstruction of the evolved phenotype. In addition to single-nucleotide mutations, a whole-genome duplication event had occurred in both evolved multicellular strains. Construction of a diploid reference strain with two mutant ace2 alleles led to complete reconstruction of the multicellular-fast sedimenting phenotype. This study shows that whole-genome duplication and a frameshift mutation in ACE2 are sufficient to generate a fast-sedimenting, multicellular phenotype in S. cerevisiae. The nature of the ace2 mutations and their occurrence in two independent evolution experiments encompassing fewer than 500 generations of selective growth suggest that switching between unicellular and multicellular phenotypes may be relevant for competitiveness of S. cerevisiae in natural environments.
Keywords: reverse engineering; whole genome sequencing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Laboratory Evolution of a Biotin-Requiring Saccharomyces cerevisiae Strain for Full Biotin Prototrophy and Identification of Causal Mutations.Appl Environ Microbiol. 2017 Aug 1;83(16):e00892-17. doi: 10.1128/AEM.00892-17. Print 2017 Aug 15. Appl Environ Microbiol. 2017. PMID: 28600311 Free PMC article.
-
A Case Study of Genomic Instability in an Industrial Strain of Saccharomyces cerevisiae.G3 (Bethesda). 2018 Nov 6;8(11):3703-3713. doi: 10.1534/g3.118.200446. G3 (Bethesda). 2018. PMID: 30254181 Free PMC article.
-
Adaptive Laboratory Evolution and Reverse Engineering of Single-Vitamin Prototrophies in Saccharomyces cerevisiae.Appl Environ Microbiol. 2020 Jun 2;86(12):e00388-20. doi: 10.1128/AEM.00388-20. Print 2020 Jun 2. Appl Environ Microbiol. 2020. PMID: 32303542 Free PMC article.
-
Experimental evolution of the model eukaryote Saccharomyces cerevisiae yields insight into the molecular mechanisms underlying adaptation.Curr Opin Microbiol. 2015 Dec;28:1-9. doi: 10.1016/j.mib.2015.06.018. Epub 2015 Jul 17. Curr Opin Microbiol. 2015. PMID: 26202939 Review.
-
Yeast as a Tool to Understand the Significance of Human Disease-Associated Gene Variants.Genes (Basel). 2021 Aug 24;12(9):1303. doi: 10.3390/genes12091303. Genes (Basel). 2021. PMID: 34573285 Free PMC article. Review.
Cited by
-
Apoptosis in snowflake yeast: novel trait, or side effect of toxic waste?J R Soc Interface. 2016 May;13(118):20160121. doi: 10.1098/rsif.2016.0121. J R Soc Interface. 2016. PMID: 27146690 Free PMC article.
-
Elucidating aromatic acid tolerance at low pH in Saccharomyces cerevisiae using adaptive laboratory evolution.Proc Natl Acad Sci U S A. 2020 Nov 10;117(45):27954-27961. doi: 10.1073/pnas.2013044117. Epub 2020 Oct 26. Proc Natl Acad Sci U S A. 2020. PMID: 33106428 Free PMC article.
-
Adaptation and phenotypic diversification of Bacillus thuringiensis biofilm are accompanied by fuzzy spreader morphotypes.NPJ Biofilms Microbiomes. 2022 Apr 13;8(1):27. doi: 10.1038/s41522-022-00292-1. NPJ Biofilms Microbiomes. 2022. PMID: 35418164 Free PMC article.
-
Experimental Evolution Reveals Favored Adaptive Routes to Cell Aggregation in Yeast.Genetics. 2017 Jun;206(2):1153-1167. doi: 10.1534/genetics.116.198895. Epub 2017 Apr 26. Genetics. 2017. PMID: 28450459 Free PMC article.
-
Diverse conditions support near-zero growth in yeast: Implications for the study of cell lifespan.Microb Cell. 2019 Aug 20;6(9):397-413. doi: 10.15698/mic2019.09.690. Microb Cell. 2019. PMID: 31528631 Free PMC article. Review.
References
-
- Sauer U. Evolutionary engineering of industrially important microbial phenotypes. Adv Biochem Eng Biotechnol. 2001;73:129–169. - PubMed
-
- Kuyper M, et al. Evolutionary engineering of mixed-sugar utilization by a xylose-fermenting Saccharomyces cerevisiae strain. FEMS Yeast Res. 2005;5(10):925–934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

