Plus-end tracking proteins, CLASPs, and a viral Akt mimic regulate herpesvirus-induced stable microtubule formation and virus spread
- PMID: 24145430
- PMCID: PMC3831478
- DOI: 10.1073/pnas.1310760110
Plus-end tracking proteins, CLASPs, and a viral Akt mimic regulate herpesvirus-induced stable microtubule formation and virus spread
Abstract
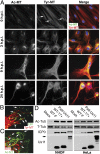
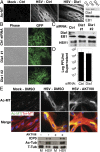
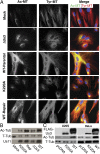
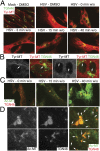
Although microtubules (MTs) frequently form highly dynamic networks, subsets of MTs become stabilized in response to environmental cues and function as specialized tracks for vesicle and macromolecular trafficking. MT stabilization is controlled by specialized plus-end tracking proteins (+TIPs) whose accumulation at the MT ends is facilitated by the end-binding protein, EB1, and regulated by various signaling pathways. As cargoes themselves, viruses are dependent on MTs for their intracellular movement. Although many viruses affect MT organization, the potential contribution of MT stabilization by +TIPs to infection remains unknown. Here we show that early in infection of primary human fibroblasts, herpes simplex virus type 1 (HSV-1) disrupts the centrosome, the primary MT organizing center in many cell types. As infection progresses HSV-1 induces the formation of stable MT subsets through inactivation of glycogen synthase kinase 3beta by the viral Ser/Thr kinase, Us3. Stable MT formation is reduced in cells infected with Us3 mutants and those stable MTs that form cluster around the trans-Golgi network. Downstream of glycogen synthase kinase 3beta, cytoplasmic linker-associated proteins (CLASPs), specialized host +TIPs that control MT formation at the trans-Golgi network and cortical capture, are specifically required for virus-induced MT stabilization and HSV-1 spread. Our findings demonstrate the biological importance of +TIPs to viral infection and suggest that HSV-1 has evolved to exploit the trans-Golgi network as an alternate MT organizing center to facilitate virus spread.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Human Cytomegalovirus Exploits TACC3 To Control Microtubule Dynamics and Late Stages of Infection.J Virol. 2021 Aug 25;95(18):e0082121. doi: 10.1128/JVI.00821-21. Epub 2021 Aug 25. J Virol. 2021. PMID: 34191581 Free PMC article.
-
HIV-1 capsid exploitation of the host microtubule cytoskeleton during early infection.Retrovirology. 2021 Jul 6;18(1):19. doi: 10.1186/s12977-021-00563-3. Retrovirology. 2021. PMID: 34229718 Free PMC article. Review.
-
GSK3beta phosphorylation modulates CLASP-microtubule association and lamella microtubule attachment.J Cell Biol. 2009 Mar 23;184(6):895-908. doi: 10.1083/jcb.200901042. Epub 2009 Mar 16. J Cell Biol. 2009. PMID: 19289791 Free PMC article.
-
The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts.Mol Biol Cell. 2006 Dec;17(12):5004-16. doi: 10.1091/mbc.e05-10-0914. Epub 2006 Sep 20. Mol Biol Cell. 2006. PMID: 16987962 Free PMC article.
-
Microtubule Regulation and Function during Virus Infection.J Virol. 2017 Jul 27;91(16):e00538-17. doi: 10.1128/JVI.00538-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28615197 Free PMC article. Review.
Cited by
-
Regulation of alphaherpesvirus protein via post-translational phosphorylation.Vet Res. 2022 Nov 17;53(1):93. doi: 10.1186/s13567-022-01115-z. Vet Res. 2022. PMID: 36397147 Free PMC article. Review.
-
Cellular mechanisms of alpha herpesvirus egress: live cell fluorescence microscopy of pseudorabies virus exocytosis.PLoS Pathog. 2014 Dec 4;10(12):e1004535. doi: 10.1371/journal.ppat.1004535. eCollection 2014 Dec. PLoS Pathog. 2014. PMID: 25474634 Free PMC article.
-
Pseudorabies Virus US3-Induced Tunneling Nanotubes Contain Stabilized Microtubules, Interact with Neighboring Cells via Cadherins, and Allow Intercellular Molecular Communication.J Virol. 2017 Sep 12;91(19):e00749-17. doi: 10.1128/JVI.00749-17. Print 2017 Oct 1. J Virol. 2017. PMID: 28747498 Free PMC article.
-
The role of PI3K/Akt in human herpesvirus infection: From the bench to the bedside.Virology. 2015 May;479-480:568-77. doi: 10.1016/j.virol.2015.02.040. Epub 2015 Mar 20. Virology. 2015. PMID: 25798530 Free PMC article. Review.
-
Herpes Simplex Virus Type 1 Neuronal Infection Triggers the Disassembly of Key Structural Components of Dendritic Spines.Front Cell Neurosci. 2021 Feb 23;15:580717. doi: 10.3389/fncel.2021.580717. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33708072 Free PMC article.
References
-
- Li R, Gundersen GG. Beyond polymer polarity: How the cytoskeleton builds a polarized cell. Nat Rev Mol Cell Biol. 2008;9(11):860–873. - PubMed
-
- Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: Mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12(12):773–786. - PubMed
-
- Gouveia SM, Akhmanova A. (2010) Cell and molecular biology of microtubule plus end tracking proteins: End binding proteins and their partners. Int Rev Cell Mol Biol. 285:1–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

