Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism
- PMID: 24145431
- PMCID: PMC3831479
- DOI: 10.1073/pnas.1317049110
Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism
Abstract
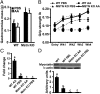
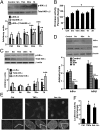
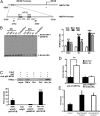
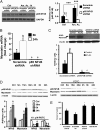
Loss of muscle mass, or sarcopenia, is nearly universal in cirrhosis and adversely affects patient outcome. The underlying cross-talk between the liver and skeletal muscle mediating sarcopenia is not well understood. Hyperammonemia is a consistent abnormality in cirrhosis due to impaired hepatic detoxification to urea. We observed elevated levels of ammonia in both plasma samples and skeletal muscle biopsies from cirrhotic patients compared with healthy controls. Furthermore, skeletal muscle from cirrhotics had increased expression of myostatin, a known inhibitor of skeletal muscle accretion and growth. In vivo studies in mice showed that hyperammonemia reduced muscle mass and strength and increased myostatin expression in wild-type compared with postdevelopmental myostatin knockout mice. We postulated that hyperammonemia is an underlying link between hepatic dysfunction in cirrhosis and skeletal muscle loss. Therefore, murine C2C12 myotubes were treated with ammonium acetate resulting in intracellular concentrations similar to those in cirrhotic muscle. In this system, we demonstrate that hyperammonemia stimulated myostatin expression in a NF-κB-dependent manner. This finding was also observed in primary murine muscle cell cultures. Hyperammonemia triggered activation of IκB kinase, NF-κB nuclear translocation, binding of the NF-κB p65 subunit to specific sites within the myostatin promoter, and stimulation of myostatin gene transcription. Pharmacologic inhibition or gene silencing of NF-κB abolished myostatin up-regulation under conditions of hyperammonemia. Our work provides unique insights into hyperammonemia-induced myostatin expression and suggests a mechanism by which sarcopenia develops in cirrhotic patients.
Keywords: portosystemic shunting; signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Ammonia elicits a different myogenic response in avian and murine myotubes.In Vitro Cell Dev Biol Anim. 2017 Feb;53(2):99-110. doi: 10.1007/s11626-016-0088-z. Epub 2016 Aug 29. In Vitro Cell Dev Biol Anim. 2017. PMID: 27573411
-
Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis.Am J Physiol Endocrinol Metab. 2012 Oct 15;303(8):E983-93. doi: 10.1152/ajpendo.00183.2012. Epub 2012 Aug 14. Am J Physiol Endocrinol Metab. 2012. PMID: 22895779 Free PMC article.
-
Ammonia lowering reverses sarcopenia of cirrhosis by restoring skeletal muscle proteostasis.Hepatology. 2017 Jun;65(6):2045-2058. doi: 10.1002/hep.29107. Epub 2017 Apr 28. Hepatology. 2017. PMID: 28195332 Free PMC article.
-
Hyperammonemia and proteostasis in cirrhosis.Curr Opin Clin Nutr Metab Care. 2018 Jan;21(1):30-36. doi: 10.1097/MCO.0000000000000426. Curr Opin Clin Nutr Metab Care. 2018. PMID: 29035972 Free PMC article. Review.
-
Sarcopenia in cirrhosis: from pathogenesis to interventions.J Gastroenterol. 2019 Oct;54(10):845-859. doi: 10.1007/s00535-019-01605-6. Epub 2019 Aug 7. J Gastroenterol. 2019. PMID: 31392488 Free PMC article. Review.
Cited by
-
Sarcopenia in Chronic Liver Disease: A Metabolic Perspective.J Clin Transl Hepatol. 2022 Dec 28;10(6):1213-1222. doi: 10.14218/JCTH.2022.00239. Epub 2022 Aug 9. J Clin Transl Hepatol. 2022. PMID: 36381104 Free PMC article. Review.
-
Liver transplantation and alcoholic liver disease: History, controversies, and considerations.World J Gastroenterol. 2018 Jul 14;24(26):2785-2805. doi: 10.3748/wjg.v24.i26.2785. World J Gastroenterol. 2018. PMID: 30018475 Free PMC article. Review.
-
Causes of Sarcopenia in Liver Cirrhosis.Clin Liver Dis (Hoboken). 2019 Dec 20;14(5):167-170. doi: 10.1002/cld.851. eCollection 2019 Nov. Clin Liver Dis (Hoboken). 2019. PMID: 31879557 Free PMC article. Review. No abstract available.
-
Fatty Hepatocytes Induce Skeletal Muscle Atrophy In Vitro: A New 3D Platform to Study the Protective Effect of Albumin in Non-Alcoholic Fatty Liver.Biomedicines. 2022 Apr 21;10(5):958. doi: 10.3390/biomedicines10050958. Biomedicines. 2022. PMID: 35625696 Free PMC article.
-
Rifaximin enhances the L‑carnitine‑mediated preventive effects on skeletal muscle atrophy in cirrhotic rats by modulating the gut‑liver‑muscle axis.Int J Mol Med. 2022 Aug;50(2):101. doi: 10.3892/ijmm.2022.5157. Epub 2022 Jun 10. Int J Mol Med. 2022. PMID: 35686541 Free PMC article.
References
-
- Hepple RT. Muscle atrophy is not always sarcopenia. J Appl Physiol (1985) 2012;113(4):677–679. - PubMed
-
- Montano-Loza AJ, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):166–173. - PubMed
-
- Olde Damink SW, Jalan R, Dejong CH. Interorgan ammonia trafficking in liver disease. Metab Brain Dis. 2009;24(1):169–181. - PubMed
-
- Holecek M, Sprongl L, Tichý M. Effect of hyperammonemia on leucine and protein metabolism in rats. Metabolism. 2000;49(10):1330–1334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

