Recognition of synthetic glycopeptides by HIV-1 broadly neutralizing antibodies and their unmutated ancestors
- PMID: 24145434
- PMCID: PMC3831483
- DOI: 10.1073/pnas.1317855110
Recognition of synthetic glycopeptides by HIV-1 broadly neutralizing antibodies and their unmutated ancestors
Abstract
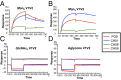
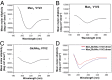
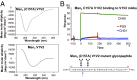
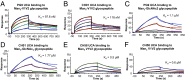
Current HIV-1 vaccines elicit strain-specific neutralizing antibodies. Broadly neutralizing antibodies (BnAbs) are not induced by current vaccines, but are found in plasma in ∼20% of HIV-1-infected individuals after several years of infection. One strategy for induction of unfavored antibody responses is to produce homogeneous immunogens that selectively express BnAb epitopes but minimally express dominant strain-specific epitopes. Here we report that synthetic, homogeneously glycosylated peptides that bind avidly to variable loop 1/2 (V1V2) BnAbs PG9 and CH01 bind minimally to strain-specific neutralizing V2 antibodies that are targeted to the same envelope polypeptide site. Both oligomannose derivatization and conformational stabilization by disulfide-linked dimer formation of synthetic V1V2 peptides were required for strong binding of V1V2 BnAbs. An HIV-1 vaccine should target BnAb unmutated common ancestor (UCA) B-cell receptors of naïve B cells, but to date no HIV-1 envelope constructs have been found that bind to the UCA of V1V2 BnAb PG9. We demonstrate herein that V1V2 glycopeptide dimers bearing Man5GlcNAc2 glycan units bind with apparent nanomolar affinities to UCAs of V1V2 BnAbs PG9 and CH01 and with micromolar affinity to the UCA of a V2 strain-specific antibody. The higher-affinity binding of these V1V2 glycopeptides to BnAbs and their UCAs renders these glycopeptide constructs particularly attractive immunogens for targeting subdominant HIV-1 envelope V1V2-neutralizing antibody-producing B cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

