BC-box protein domain-related mechanism for VHL protein degradation
- PMID: 24145437
- PMCID: PMC3831490
- DOI: 10.1073/pnas.1311382110
BC-box protein domain-related mechanism for VHL protein degradation
Abstract
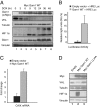
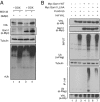
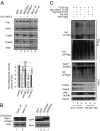
The tumor suppressor VHL (von Hippel-Lindau) protein is a substrate receptor for Ubiquitin Cullin Ring Ligase complexes (CRLs), containing a BC-box domain that associates to the adaptor Elongin B/C. VHL targets hypoxia-inducible factor 1α to proteasome-dependent degradation. Gam1 is an adenoviral protein, which also possesses a BC-box domain that interacts with the host Elongin B/C, thereby acting as a viral substrate receptor. Gam1 associates with both Cullin2 and Cullin5 to form CRL complexes targeting the host protein SUMO enzyme SAE1 for proteasomal degradation. We show that Gam1 protein expression induces VHL protein degradation leading to hypoxia-inducible factor 1α stabilization and induction of its downstream targets. We also characterize the CRL-dependent mechanism that drives VHL protein degradation via proteasome. Interestingly, expression of Suppressor of Cytokine Signaling (SOCS) domain-containing viral proteins and cellular BC-box proteins leads to VHL protein degradation, in a SOCS domain-containing manner. Our work underscores the exquisite ability of viral domains to uncover new regulatory mechanisms by hijacking key cellular proteins.
Keywords: hypoxia; oncoviral proteins; ubiquitylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
-
- Petroski MD, Deshaies RJ. Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol. 2005;6(1):9–20. - PubMed
-
- Yan Q, et al. Identification of Elongin C and Skp1 sequences that determine Cullin selection. J Biol Chem. 2004;279(41):43019–43026. - PubMed
-
- Duan DR, et al. Inhibition of transcription elongation by the VHL tumor suppressor protein. Science. 1995;269(5229):1402–1406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

