Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees
- PMID: 24145453
- PMCID: PMC3831983
- DOI: 10.1073/pnas.1314923110
Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees
Abstract
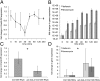
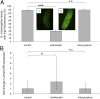
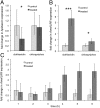
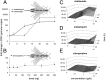
Large-scale losses of honey bee colonies represent a poorly understood problem of global importance. Both biotic and abiotic factors are involved in this phenomenon that is often associated with high loads of parasites and pathogens. A stronger impact of pathogens in honey bees exposed to neonicotinoid insecticides has been reported, but the causal link between insecticide exposure and the possible immune alteration of honey bees remains elusive. Here, we demonstrate that the neonicotinoid insecticide clothianidin negatively modulates NF-κB immune signaling in insects and adversely affects honey bee antiviral defenses controlled by this transcription factor. We have identified in insects a negative modulator of NF-κB activation, which is a leucine-rich repeat protein. Exposure to clothianidin, by enhancing the transcription of the gene encoding this inhibitor, reduces immune defenses and promotes the replication of the deformed wing virus in honey bees bearing covert infections. This honey bee immunosuppression is similarly induced by a different neonicotinoid, imidacloprid, but not by the organophosphate chlorpyriphos, which does not affect NF-κB signaling. The occurrence at sublethal doses of this insecticide-induced viral proliferation suggests that the studied neonicotinoids might have a negative effect at the field level. Our experiments uncover a further level of regulation of the immune response in insects and set the stage for studies on neural modulation of immunity in animals. Furthermore, this study has implications for the conservation of bees, as it will contribute to the definition of more appropriate guidelines for testing chronic or sublethal effects of pesticides used in agriculture.
Keywords: Apis mellifera; DWV; NLR (CLR); neuroimmunity; toxicology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- van der Zee R, et al. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008-9 and 2009-10. J Apic Res. 2012;51(1):100–114.
-
- vanEngelsdorp D, et al. A national survey of managed honey bee 2010-11 winter colony losses in the USA: Results from the Bee Informed Partnership. J Apic Res. 2012;51(1):115–124.
-
- Ratnieks FL, Carreck NL. Ecology. Clarity on honey bee collapse? Science. 2010;327(5962):152–153. - PubMed
-
- Neumann P, Carreck NL. Honeybee colony losses. J Apic Res. 2010;49(1):1–6.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

