Bioorthogonal chemistry: strategies and recent developments
- PMID: 24145483
- PMCID: PMC3847904
- DOI: 10.1039/c3cc44272a
Bioorthogonal chemistry: strategies and recent developments
Erratum in
- Chem Commun (Camb). 2014 Aug 28;50(67):9595
Abstract
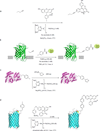
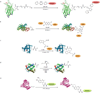
The use of covalent chemistry to track biomolecules in their native environment-a focus of bioorthogonal chemistry-has received considerable interest recently among chemical biologists and organic chemists alike. To facilitate wider adoption of bioorthogonal chemistry in biomedical research, a central effort in the last few years has been focused on the optimization of a few known bioorthogonal reactions, particularly with respect to reaction kinetics improvement, novel genetic encoding systems, and fluorogenic reactions for bioimaging. During these optimizations, three strategies have emerged, including the use of ring strain for substrate activation in the cycloaddition reactions, the discovery of new ligands and privileged substrates for accelerated metal-catalysed reactions, and the design of substrates with pre-fluorophore structures for rapid "turn-on" fluorescence after selective bioorthogonal reactions. In addition, new bioorthogonal reactions based on either modified or completely unprecedented reactant pairs have been reported. Finally, increasing attention has been directed toward the development of mutually exclusive bioorthogonal reactions and their applications in multiple labeling of a biomolecule in cell culture. In this feature article, we wish to present the recent progress in bioorthogonal reactions through the selected examples that highlight the above-mentioned strategies. Considering increasing sophistication in bioorthogonal chemistry development, we strive to project several exciting opportunities where bioorthogonal chemistry can make a unique contribution to biology in the near future.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

