CD36-specific antibodies block release of HIV-1 from infected primary macrophages and its transmission to T cells
- PMID: 24145510
- PMCID: PMC3832921
- DOI: 10.1084/jem.20130566
CD36-specific antibodies block release of HIV-1 from infected primary macrophages and its transmission to T cells
Abstract
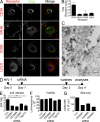
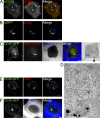
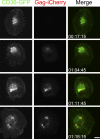
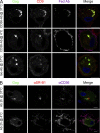
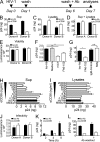
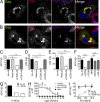
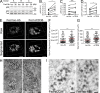
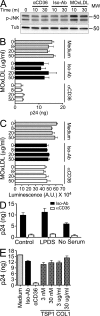
HIV-1-infected macrophages likely represent viral reservoirs, as they accumulate newly formed virions in internal virus-containing compartments (VCCs). However, the nature and biogenesis of VCCs remain poorly defined. We show that upon HIV-1 infection of primary human macrophages, Gag is recruited to preexisting compartments containing the scavenger receptor CD36, which then become VCCs. Silencing of CD36 in HIV-1-infected macrophages decreases the amount of virions released. Strikingly, soluble anti-CD36 antibodies, but not the natural ligands of CD36, inhibit release of virions from HIV-1-infected macrophages and the transmission of virus to CD4(+) T cells. The effect of the antibodies is potent, rapid, and induces the retention of virions within VCCs. Ectopic expression of CD36 in HeLa cells renders them susceptible to the inhibitory effect of the anti-CD36 mAb upon HIV-1 infection. We show that the anti-CD36 mAb inhibits HIV-1 release by clustering newly formed virions at their site of budding, and that signaling via CD36 is not required. Thus, HIV-1 reservoirs in macrophages may be tackled therapeutically using anti-CD36 antibodies to prevent viral dissemination.
Figures

References
-
- Bennett A.E., Narayan K., Shi D., Hartnell L.M., Gousset K., He H., Lowekamp B.C., Yoo T.S., Bliss D., Freed E.O., Subramaniam S. 2009. Ion-abrasion scanning electron microscopy reveals surface-connected tubular conduits in HIV-infected macrophages. PLoS Pathog. 5:e1000591 10.1371/journal.ppat.1000591 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

