Overexpression of TLR7 promotes cell-intrinsic expansion and autoantibody production by transitional T1 B cells
- PMID: 24145511
- PMCID: PMC3832927
- DOI: 10.1084/jem.20122798
Overexpression of TLR7 promotes cell-intrinsic expansion and autoantibody production by transitional T1 B cells
Abstract
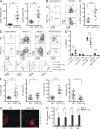
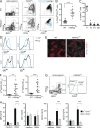
Toll-like receptor (TLR), a ligand for single-stranded RNA, has been implicated in the development of pathogenic anti-RNA autoantibodies both in systemic lupus erythematous (SLE) patients and in murine models of lupus. It is still unclear, however, where and how TLR7-mediated interactions affect the development of autoreactive B cells. We found that overexpression of TLR7 in transgenic mice (TLR7.1Tg) leads to marked alterations of transitional (T1) B cells, associated with their expansion and proliferation within the splenic red pulp (RP). This phenotype was intrinsic to the T1 subset of B cells and occurred independently of type 1 IFN signals. Overexpression of RNase in TLR7.1Tg mice significantly limited the expansion and proliferation of T1 cells, indicating that endogenous RNA complexes are driving their activation. TLR7.1Tg T1 cells were hyper-responsive to anti-IgM and TLR7 ligand stimulation in vitro and produced high concentrations of class-switched IgG2b and IgG2c, including anti-RNA antibodies. Our results demonstrate that initial TLR7 stimulation of B cells occurs at the T1 stage of differentiation in the splenic RP and suggest that dysregulation of TLR7 expression in T1 cells can result in production of autoantibodies.
Figures





Similar articles
-
Integrated B Cell, Toll-like, and BAFF Receptor Signals Promote Autoantibody Production by Transitional B Cells.J Immunol. 2018 Dec 1;201(11):3258-3268. doi: 10.4049/jimmunol.1800393. Epub 2018 Oct 29. J Immunol. 2018. PMID: 30373855 Free PMC article.
-
High TLR7 Expression Drives the Expansion of CD19+CD24hiCD38hi Transitional B Cells and Autoantibody Production in SLE Patients.Front Immunol. 2019 Jun 4;10:1243. doi: 10.3389/fimmu.2019.01243. eCollection 2019. Front Immunol. 2019. PMID: 31231380 Free PMC article.
-
B cell autophagy mediates TLR7-dependent autoimmunity and inflammation.Autophagy. 2015;11(7):1010-24. doi: 10.1080/15548627.2015.1052206. Autophagy. 2015. PMID: 26120731 Free PMC article.
-
Emerging roles of TLR7 and TLR9 in murine SLE.J Autoimmun. 2009 Nov-Dec;33(3-4):231-8. doi: 10.1016/j.jaut.2009.10.001. Epub 2009 Oct 21. J Autoimmun. 2009. PMID: 19846276 Review.
-
Characterization of splenic CD21hi T2 B cells.Immunol Res. 2007;39(1-3):240-8. doi: 10.1007/s12026-007-0072-5. Immunol Res. 2007. PMID: 17917068 Review.
Cited by
-
Autoimmune B Cell Repertoire in a Mouse Model of Sjögren's Syndrome.Front Immunol. 2021 Apr 23;12:666545. doi: 10.3389/fimmu.2021.666545. eCollection 2021. Front Immunol. 2021. PMID: 33968069 Free PMC article.
-
B cell-activating factor (BAFF) from dendritic cells, monocytes and neutrophils is required for B cell maturation and autoantibody production in SLE-like autoimmune disease.Front Immunol. 2023 Feb 27;14:1050528. doi: 10.3389/fimmu.2023.1050528. eCollection 2023. Front Immunol. 2023. PMID: 36923413 Free PMC article.
-
CD22: A Regulator of Innate and Adaptive B Cell Responses and Autoimmunity.Front Immunol. 2018 Sep 28;9:2235. doi: 10.3389/fimmu.2018.02235. eCollection 2018. Front Immunol. 2018. PMID: 30323814 Free PMC article. Review.
-
RIP1/RIP3-regulated necroptosis as a target for multifaceted disease therapy (Review).Int J Mol Med. 2019 Sep;44(3):771-786. doi: 10.3892/ijmm.2019.4244. Epub 2019 Jun 14. Int J Mol Med. 2019. PMID: 31198981 Free PMC article. Review.
-
Sources of Pathogenic Nucleic Acids in Systemic Lupus Erythematosus.Front Immunol. 2019 May 8;10:1028. doi: 10.3389/fimmu.2019.01028. eCollection 2019. Front Immunol. 2019. PMID: 31139185 Free PMC article. Review.
References
-
- Amano H., Amano E., Moll T., Marinkovic D., Ibnou-Zekri N., Martinez-Soría E., Semac I., Wirth T., Nitschke L., Izui S. 2003. The Yaa mutation promoting murine lupus causes defective development of marginal zone B cells. J. Immunol. 170:2293–2301 - PubMed
-
- Beisner D.R., Langerak P., Parker A.E., Dahlberg C., Otero F.J., Sutton S.E., Poirot L., Barnes W., Young M.A., Niessen S., et al. 2013. The intramembrane protease Sppl2a is required for B cell and DC development and survival via cleavage of the invariant chain. J. Exp. Med. 210:23–30 10.1084/jem.20121072 - DOI - PMC - PubMed
-
- Bekeredjian-Ding I.B., Wagner M., Hornung V., Giese T., Schnurr M., Endres S., Hartmann G. 2005. Plasmacytoid dendritic cells control TLR7 sensitivity of naive B cells via type I IFN. J. Immunol. 174:4043–4050 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

