Ganciclovir-resistant cytomegalovirus infections among lung transplant recipients are associated with poor outcomes despite treatment with foscarnet-containing regimens
- PMID: 24145525
- PMCID: PMC3910731
- DOI: 10.1128/AAC.00561-13
Ganciclovir-resistant cytomegalovirus infections among lung transplant recipients are associated with poor outcomes despite treatment with foscarnet-containing regimens
Abstract
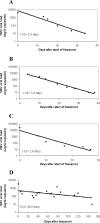
Ganciclovir-resistant cytomegalovirus (CMV) infections are reported infrequently among lung transplant recipients receiving extended valganciclovir prophylaxis. We performed a single-center, retrospective review of ganciclovir-resistant CMV infections in a program that employed valganciclovir prophylaxis for ≥6 months after lung transplant. CMV infections were diagnosed in 28% (170/607) of patients. UL97 mutations were detected in 9.4% (16/170) of CMV-infected patients at a median of 8.5 months posttransplant (range, 5 to 21) and despite prophylaxis for a median of 7 months (range, 4 to 21). UL97 mutations were canonical; 25% (4/16) of strains carried concurrent UL54 mutations. Ganciclovir-resistant CMV was more likely with breakthrough infections (75% [12/16] versus 19% [30/154]; P = 0.00001) and donor positive/recipient negative (D+/R-) serostatus (75% versus 45% [69/154]; P = 0.03). The median whole-blood CMV load was 4.13 log10 copies/cm(3) (range, 2.54 to 5.53), and 93% (14/15) of patients had low-moderate immune responses (Cylex Immunoknow). Antiviral therapy was successful, failed, or eradicated viremia followed by relapse in 12% (2/16), 31% (5/16), and 56% (9/16) of patients, respectively. Eighty-seven percent (14/16) of patients were treated with foscarnet-containing regimens; toxicity developed in 78% (11/14) of these. Median viral load half-life and time to viremia eradication among foscarnet-treated patients were 2.6 and 23 days, respectively, and did not correlate with protection from relapse. Sixty-nine percent (11/16) of patients developed CMV pneumonitis, and 25% (4/16) died of it. Serum viral load was independently associated with death among foscarnet-treated patients (P = 0.04). In conclusion, ganciclovir-resistant CMV infections remained a major cause of morbidity and mortality following lung transplantation. Foscarnet-based regimens often eradicated viremia rapidly but were ineffective in the long term and limited by toxicity.
Figures
References
-
- Ettinger NA, Bailey TC, Trulock EP, Storch GA, Anderson D, Raab S, Spitznagel EL, Dresler C, Cooper JD. 1993. Cytomegalovirus infection and pneumonitis. Impact after isolated lung transplantation. Washington University Lung Transplant Group. Am. Rev. Respir. Dis. 147:1017–1023 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

