Novel antifungal drug discovery based on targeting pathways regulating the fungus-conserved Upc2 transcription factor
- PMID: 24145546
- PMCID: PMC3910738
- DOI: 10.1128/AAC.01677-13
Novel antifungal drug discovery based on targeting pathways regulating the fungus-conserved Upc2 transcription factor
Abstract
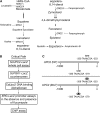
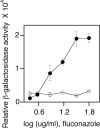
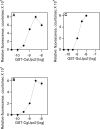
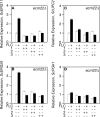
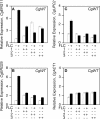
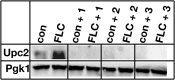
Infections by Candida albicans and related fungal pathogens pose a serious health problem for immunocompromised patients. Azole drugs, the most common agents used to combat infections, target the sterol biosynthetic pathway. Adaptation to azole therapy develops as drug-stressed cells compensate by upregulating several genes in the pathway, a process mediated in part by the Upc2 transcription factor. We have implemented a cell-based high-throughput screen to identify small-molecule inhibitors of Upc2-dependent induction of sterol gene expression in response to azole drug treatment. The assay is designed to identify not only Upc2 DNA binding inhibitors but also compounds impeding the activation of gene expression by Upc2. An AlphaScreen assay was developed to determine whether the compounds identified interact directly with Upc2 and inhibit DNA binding. Three compounds identified by the cell-based assay inhibited Upc2 protein level and UPC2-LacZ gene expression in response to a block in sterol biosynthesis. The compounds were growth inhibitory and attenuated antifungal-induced sterol gene expression in vivo. They did so by reducing the level of Upc2 protein and Upc2 DNA binding in the presence of drug. The mechanism by which the compounds restrict Upc2 DNA binding is not through a direct interaction, as demonstrated by a lack of DNA binding inhibitory activity using the AlphaScreen assay. Rather, they likely inhibit a novel pathway activating Upc2 in response to a block in sterol biosynthesis. We suggest that the compounds identified represent potential precursors for the synthesis of novel antifungal drugs.
Figures


References
-
- Pfaller MA, Jones RN, Doern GV, Sader HS, Messer SA, Houston A, Coffman S, Hollis RJ. 2000. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997–1998. Antimicrob. Agents Chemother. 44:747–751. 10.1128/AAC.44.3.747-751.2000 - DOI - PMC - PubMed
-
- Vazquez JA, Sobel JD, Peng G, Steele-Moore L, Schuman P, Holloway W, Neaton JD. 1999. Evolution of vaginal Candida species recovered from human immunodeficiency virus-infected women receiving fluconazole prophylaxis: the emergence of Candida glabrata? Terry Beirn Community Programs for Clinical Research in AIDS (CPCRA). Clin. Infect. Dis. 28:1025–1031 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

