Preparation and characterization of an advanced medical device for bone regeneration
- PMID: 24146118
- PMCID: PMC3909167
- DOI: 10.1208/s12249-013-0033-3
Preparation and characterization of an advanced medical device for bone regeneration
Abstract
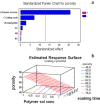
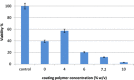
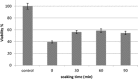
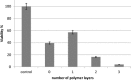
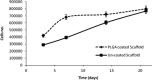
Tridimensional scaffolds can promote bone regeneration as a framework supporting the migration of cells from the surrounding tissue into the damaged tissue and as delivery systems for the controlled or prolonged release of cells, genes, and growth factors. The goal of the work was to obtain an advanced medical device for bone regeneration through coating a decellularized and deproteinized bone matrix of bovine origin with a biodegradable, biocompatible polymer, to improve the cell engraftment on the bone graft. The coating protocol was studied and set up to obtain a continuous and homogeneous polylactide-co-glycolide (PLGA) coating on the deproteinized bone matrix Orthoss® block without occluding pores and decreasing the scaffold porosity. The PLGA-coated scaffolds were characterized for their morphology and porosity. The effects of PLGA polymer coating on cell viability were assessed with the 3-(4,5-dimethyl-2-thiazolyl)-2,5 diphenyl-2H-tetrazolium assay. The polymer solution concentration and the number of polymeric layers were the main variables affecting coating efficiency and porosity of the original decellularized bone matrix. The designed polymer coating protocol did not affect the trabecular structure of the original decellularized bone matrix. The PLGA-coated decellularized bone matrix maintained the structural features, and it improved the ability in stimulating fibroblasts attachment and proliferation.
Figures


Similar articles
-
Control of pore size and structure of tissue engineering scaffolds produced by supercritical fluid processing.Eur Cell Mater. 2007 Dec 17;14:64-77. doi: 10.22203/ecm.v014a07. Eur Cell Mater. 2007. PMID: 18085505
-
Design, fabrication, and characterization of a composite scaffold for bone tissue engineering.Int J Artif Organs. 2008 Aug;31(8):697-707. doi: 10.1177/039139880803100803. Int J Artif Organs. 2008. PMID: 18825642
-
In vivo characterisation of a novel bioresorbable poly(lactide-co-glycolide) tubular foam scaffold for tissue engineering applications.J Mater Sci Mater Med. 2004 Jun;15(6):729-34. doi: 10.1023/b:jmsm.0000030216.73274.86. J Mater Sci Mater Med. 2004. PMID: 15346742
-
Performance comparison of PLA- and PLGA-coated porous bioceramic scaffolds: Mechanical, biodegradability, bioactivity, delivery and biocompatibility assessments.J Control Release. 2022 Nov;351:1-7. doi: 10.1016/j.jconrel.2022.09.022. Epub 2022 Sep 18. J Control Release. 2022. PMID: 36115555 Review.
-
Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration.Mater Sci Eng C Mater Biol Appl. 2020 May;110:110698. doi: 10.1016/j.msec.2020.110698. Epub 2020 Jan 29. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32204012 Free PMC article. Review.
Cited by
-
Recent Approaches to the Manufacturing of Biomimetic Multi-Phasic Scaffolds for Osteochondral Regeneration.Int J Mol Sci. 2018 Jun 13;19(6):1755. doi: 10.3390/ijms19061755. Int J Mol Sci. 2018. PMID: 29899285 Free PMC article. Review.
-
Bisphosphonate-incorporated coatings for orthopedic implants functionalization.Mater Today Bio. 2023 Jul 22;22:100737. doi: 10.1016/j.mtbio.2023.100737. eCollection 2023 Oct. Mater Today Bio. 2023. PMID: 37576870 Free PMC article. Review.
-
Biomaterials for Soft Tissue Repair and Regeneration: A Focus on Italian Research in the Field.Pharmaceutics. 2021 Aug 26;13(9):1341. doi: 10.3390/pharmaceutics13091341. Pharmaceutics. 2021. PMID: 34575417 Free PMC article. Review.
References
-
- Cordonnier T, Sohier J, Rosset P, Layrolle P. Biomimetic materials for bone tissue engineering—state of the art and future. Trends Adv Eng Mater. 2011;13(5):B135–B150. doi: 10.1002/adem.201080098. - DOI
-
- Zilbermann M. Studies in mechanobiology, active implants and scaffold for tissue regeneration, part ii scaffolds for bone regeneration. In: Springer editor. Tissue engineering and biomaterials, vol. 8. Berlin: Springer; 2011. pp. 169–285. doi:10.1007/978-3-642-18065-1.
-
- Schlickewei W, Schlickewei C. The use of bone substitutes in the treatment of bone defects—the clinical view and history. Macromol Symp. 2007;253:10–23. doi: 10.1002/masy.200750702. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

