Scleral structural alterations associated with chronic experimental intraocular pressure elevation in mice
- PMID: 24146537
- PMCID: PMC3783364
Scleral structural alterations associated with chronic experimental intraocular pressure elevation in mice
Abstract
Purpose: To study changes in scleral structure induced by chronic experimental intraocular pressure elevation in mice.
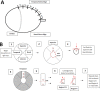
Methods: We studied the effect of chronic bead-induced glaucoma on scleral thickness, collagen lamellar structure, and collagen fibril diameter distribution in C57BL/6 (B6) and CD1 mice, and in collagen 8α2 mutant mice (Aca23) and their wild-type littermates (Aca23-WT) using electron and confocal microscopy.
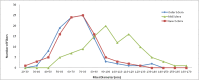
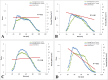
Results: In unfixed tissue, the control B6 peripapillary sclera was thicker than in CD1 mice (p<0.001). After 6 weeks of glaucoma, the unfixed CD1 and B6 sclera thinned by 9% and 12%, respectively (p<0.001). The fixed sclera, measured by electron microscopy, was significantly thicker in control Aca23 than in B6 or CD1 mice (p<0.05). The difference between fresh and fixed scleral thickness was nearly 68% in untreated control B6 and CD1 mice, but differed by only 10% or less in fresh/fixed glaucoma scleral comparisons. There were 39.3±9.6 lamellae (mean, standard deviation) in control sclera, categorized as 41% cross-section, 24% cellular, 20% oblique, and 15% longitudinal. After glaucoma, mean peripapillary thickness significantly increased in fixed specimens of all mouse strains by 10.3 ±4.8 µm (p=0.001) and the total number of lamellae increased by 18% (p=0.01). The number of cellular and cross-section lamellae increased in glaucoma eyes. After glaucoma, there were more small and fewer large collagen fibrils (p<0.0001). Second harmonic generation imaging showed that the normal circumferential pattern of collagen fibrils in the peripapillary sclera was altered in significantly damaged glaucomatous eyes.
Conclusions: Dynamic responses of the sclera to experimental mouse glaucoma may be more important than baseline anatomic features in explaining susceptibility to damage. These include decreases in nonfibrillar elements, alterations in lamellar orientation, an increased number of smaller collagen fibrils and fewer larger fibrils, and relative increase in the number of scleral fibroblast layers.
Figures




Similar articles
-
Studies of scleral biomechanical behavior related to susceptibility for retinal ganglion cell loss in experimental mouse glaucoma.Invest Ophthalmol Vis Sci. 2013 Mar 11;54(3):1767-80. doi: 10.1167/iovs.12-10952. Invest Ophthalmol Vis Sci. 2013. PMID: 23404116 Free PMC article.
-
Mice with an induced mutation in collagen 8A2 develop larger eyes and are resistant to retinal ganglion cell damage in an experimental glaucoma model.Mol Vis. 2012;18:1093-106. Epub 2012 May 1. Mol Vis. 2012. PMID: 22701298 Free PMC article.
-
Scleral permeability varies by mouse strain and is decreased by chronic experimental glaucoma.Invest Ophthalmol Vis Sci. 2014 Apr 21;55(4):2564-73. doi: 10.1167/iovs.13-13327. Invest Ophthalmol Vis Sci. 2014. PMID: 24557355 Free PMC article.
-
Development of diagnostic and treatment strategies for glaucoma through understanding and modification of scleral and lamina cribrosa connective tissue.Cell Tissue Res. 2013 Aug;353(2):231-44. doi: 10.1007/s00441-013-1603-0. Epub 2013 Mar 28. Cell Tissue Res. 2013. PMID: 23535950 Free PMC article. Review.
-
Corneal and scleral collagens--a microscopist's perspective.Micron. 2001 Apr;32(3):261-72. doi: 10.1016/s0968-4328(00)00041-x. Micron. 2001. PMID: 11006506 Review.
Cited by
-
The Shape of Posterior Sclera as a Biometric Signature in Open-angle Glaucoma: An Intereye Comparison Study.J Glaucoma. 2020 Oct;29(10):890-898. doi: 10.1097/IJG.0000000000001573. J Glaucoma. 2020. PMID: 32555059 Free PMC article.
-
Analysis of 2-dimensional regional differences in the peripapillary scleral fibroblast cytoskeleton of normotensive and hypertensive mouse eyes.Sci Rep. 2025 Jul 1;15(1):21207. doi: 10.1038/s41598-025-08251-4. Sci Rep. 2025. PMID: 40595239 Free PMC article.
-
Identification of key genes and pathways in scleral extracellular matrix remodeling in glaucoma: Potential therapeutic agents discovered using bioinformatics analysis.Int J Med Sci. 2021 Feb 4;18(7):1554-1565. doi: 10.7150/ijms.52846. eCollection 2021. Int J Med Sci. 2021. PMID: 33746571 Free PMC article.
-
Rho-Kinase Inhibition Reduces Myofibroblast Differentiation and Proliferation of Scleral Fibroblasts Induced by Transforming Growth Factor β and Experimental Glaucoma.Transl Vis Sci Technol. 2018 Nov 14;7(6):6. doi: 10.1167/tvst.7.6.6. eCollection 2018 Nov. Transl Vis Sci Technol. 2018. PMID: 30479877 Free PMC article.
-
Regional Gene Expression in the Retina, Optic Nerve Head, and Optic Nerve of Mice with Optic Nerve Crush and Experimental Glaucoma.Int J Mol Sci. 2023 Sep 6;24(18):13719. doi: 10.3390/ijms241813719. Int J Mol Sci. 2023. PMID: 37762022 Free PMC article.
References
-
- Anderson DR, Hendrickson A. Effect of intraocular pressure on rapid axoplasmic transport in monkey optic nerve. Invest Ophthalmol. 1974;13:771–83. - PubMed
-
- Quigley HA, Addicks EM, Green WR, Maumenee AE. Optic nerve damage in human glaucoma,II. The site of injury and susceptibility to damage. Arch Ophthalmol. 1981;99:635–49. - PubMed
-
- Foster CS, Sainz de la Maza M. The Sclera, Chapter 1: Structural Considerations of the Sclera. Springer Science Business Media, LLC; 2012; 1–30.
-
- Rada JAS, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82:185–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
