Alterations of cAMP-dependent signaling in dystrophic skeletal muscle
- PMID: 24146652
- PMCID: PMC3797997
- DOI: 10.3389/fphys.2013.00290
Alterations of cAMP-dependent signaling in dystrophic skeletal muscle
Abstract
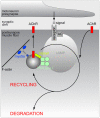
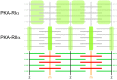
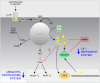
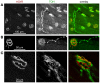
Autonomic regulation processes in striated muscles are largely mediated by cAMP/PKA-signaling. In order to achieve specificity of signaling its spatial-temporal compartmentation plays a critical role. We discuss here how specificity of cAMP/PKA-signaling can be achieved in skeletal muscle by spatio-temporal compartmentation. While a microdomain containing PKA type I in the region of the neuromuscular junction (NMJ) is important for postsynaptic, activity-dependent stabilization of the nicotinic acetylcholine receptor (AChR), PKA type I and II microdomains in the sarcomeric part of skeletal muscle are likely to play different roles, including the regulation of muscle homeostasis. These microdomains are due to specific A-kinase anchoring proteins, like rapsyn and myospryn. Importantly, recent evidence indicates that compartmentation of the cAMP/PKA-dependent signaling pathway and pharmacological activation of cAMP production are aberrant in different skeletal muscles disorders. Thus, we discuss here their potential as targets for palliative treatment of certain forms of dystrophy and myasthenia. Under physiological conditions, the neuropeptide, α-calcitonin-related peptide, as well as catecholamines are the most-mentioned natural triggers for activating cAMP/PKA signaling in skeletal muscle. While the precise domains and functions of these first messengers are still under investigation, agonists of β2-adrenoceptors clearly exhibit anabolic activity under normal conditions and reduce protein degradation during atrophic periods. Past and recent studies suggest direct sympathetic innervation of skeletal muscle fibers. In summary, the organization and roles of cAMP-dependent signaling in skeletal muscle are increasingly understood, revealing crucial functions in processes like nerve-muscle interaction and muscle trophicity.
Keywords: AKAP; PKA; adrenoceptors; dystrophy; endplate; metabolism; neuromuscular junction; skeletal muscle.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

