Voltage-dependent calcium and potassium channels in retinal glial cells
- PMID: 2414667
- PMCID: PMC2693195
- DOI: 10.1038/317809a0
Voltage-dependent calcium and potassium channels in retinal glial cells
Abstract
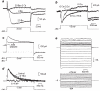
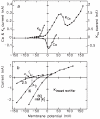
Glial cells, which outnumber neurones in the central nervous system, have traditionally been considered to be electrically inexcitable and to play only a passive role in the electrical activity of the brain. Recent reports have demonstrated, however, that certain glial cells, when maintained in primary culture, possess voltage-dependent ion channels. It remains to be demonstrated whether these channels are also present in glial cells in vivo. I show here that Müller cells, the principal glial cells of the vertebrate retina, can generate 'Ca2+ spikes' in freshly excised slices of retinal tissue. In addition, voltage-clamp studies of enzymatically dissociated Müller cells demonstrate the presence of four types of voltage-dependent ion channels: a Ca2+ channel, a Ca2+-activated K+ channel, a fast-inactivating (type A) K+ channel and an inward-rectifying K+ channel. Currents generated by these voltage-dependent channels may enhance the ability of Müller cells to regulate extracellular K+ levels in the retina and may be involved in the generation of the electroretinogram.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

