Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV® assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications
- PMID: 24146744
- PMCID: PMC3795724
- DOI: 10.1371/journal.pone.0074744
Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV® assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications
Abstract
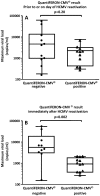
The reconstitution of anti-viral cellular immunity following hematopoietic stem cell transplantation (HSCT) is crucial in preventing cytomegalovirus (CMV)-associated complications. Thus immunological monitoring has emerged as an important tool to better target pre-emptive anti-viral therapies. However, traditional laboratory-based assays are too cumbersome and complicated to implement in a clinical setting. Here we conducted a prospective study of a new whole blood assay (referred to as QuantiFERON-CMV®) to determine the clinical utility of measuring CMV-specific CD8+ T-cell responses as a prognostic tool. Forty-one evaluable allogeneic HSCT recipients underwent weekly immunological monitoring from day 21 post-transplant and of these 21 (51.2%) showed CMV reactivation and 29 (70.7%) developed acute graft-versus-host disease (GvHD). Patients with acute GvHD (grade ≥ 2) within 6 weeks of transplant showed delayed reconstitution of CMV-specific T-cell immunity (p = 0.013) and a higher risk of CMV viremia (p = 0.026). The median time to stable CMV-specific immune reconstitution was 59 days and the incidence of CMV reactivation was lower in patients who developed this than those who did not (27% versus 65%; p = 0.031). Furthermore, a failure to reconstitute CMV-specific immunity soon after the onset of CMV viraemia was associated with higher peak viral loads (5685 copies/ml versus 875 copies/ml; p = 0.002). Hence, QuantiFERON-CMV® testing in the week following CMV viremia can be useful in identifying HSCT recipients at risk of complicated reactivation.
Conflict of interest statement
Figures



Similar articles
-
Quantiferon-Cytomegalovirus assay: A potentially useful tool in the evaluation of CMV-specific CD8+ T-cell reconstitution in pediatric hematopoietic stem cell transplant patients.Pediatr Transplant. 2018 Aug;22(5):e13220. doi: 10.1111/petr.13220. Epub 2018 May 18. Pediatr Transplant. 2018. PMID: 29777573 Clinical Trial.
-
Assessing the risk of CMV reactivation and reconstitution of antiviral immune response post bone marrow transplantation by the QuantiFERON-CMV-assay and real time PCR.J Clin Virol. 2018 Feb-Mar;99-100:61-66. doi: 10.1016/j.jcv.2018.01.002. Epub 2018 Jan 9. J Clin Virol. 2018. PMID: 29331844
-
Ex vivo monitoring of human cytomegalovirus-specific CD8(+) T-Cell responses using the QuantiFERON-CMV assay in allogeneic hematopoietic stem cell transplant recipients attending an Irish hospital.J Med Virol. 2010 Mar;82(3):433-40. doi: 10.1002/jmv.21727. J Med Virol. 2010. PMID: 20087937
-
The immune response to cytomegalovirus in allogeneic hematopoietic stem cell transplant recipients.Cell Mol Life Sci. 2015 Nov;72(21):4049-62. doi: 10.1007/s00018-015-1986-z. Epub 2015 Jul 15. Cell Mol Life Sci. 2015. PMID: 26174234 Free PMC article. Review.
-
Control of Cytomegalovirus Viremia after Allogeneic Stem Cell Transplantation: A Review on CMV-Specific T Cell Reconstitution.Biol Blood Marrow Transplant. 2018 Sep;24(9):1776-1782. doi: 10.1016/j.bbmt.2018.03.028. Epub 2018 Apr 4. Biol Blood Marrow Transplant. 2018. PMID: 29626514 Review.
Cited by
-
Adoptive T-cell therapy for pediatric cytomegalovirus-associated retinitis.Blood Adv. 2019 Jun 11;3(11):1774-1777. doi: 10.1182/bloodadvances.2019000121. Blood Adv. 2019. PMID: 31186253 Free PMC article.
-
Patient risk stratification and tailored clinical management of post-transplant CMV-, EBV-, and BKV-infections by monitoring virus-specific T-cell immunity.EJHaem. 2021 Jun 1;2(3):428-439. doi: 10.1002/jha2.175. eCollection 2021 Aug. EJHaem. 2021. PMID: 35844677 Free PMC article.
-
Immune Monitoring for CMV in Transplantation.Curr Infect Dis Rep. 2018 Mar 14;20(4):4. doi: 10.1007/s11908-018-0610-4. Curr Infect Dis Rep. 2018. PMID: 29542023 Review.
-
Low T-Cell Responses to Mitogen Stimulation Predicts Poor Survival in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation.Front Immunol. 2017 Nov 9;8:1506. doi: 10.3389/fimmu.2017.01506. eCollection 2017. Front Immunol. 2017. PMID: 29170666 Free PMC article.
-
Coinfection with Human Cytomegalovirus Genetic Variants in Transplant Recipients and Its Impact on Antiviral T Cell Immune Reconstitution.J Virol. 2016 Jul 27;90(16):7497-507. doi: 10.1128/JVI.00297-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27279616 Free PMC article.
References
-
- Gandhi MK, Khanna R (2004) Human cytomegalovirus: clinical aspects, immune regulation, and emerging treatments. Lancet Infect Dis 4: 725–738. - PubMed
-
- Boeckh M, Nichols WG (2004) The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 103: 2003–2008. - PubMed
-
- Boeckh M, Nichols WG, Papanicolaou G, Rubin R, Wingard JR, et al. (2003) Cytomegalovirus in hematopoietic stem cell transplant recipients: Current status, known challenges, and future strategies. Biol Blood Marrow Transplant 9: 543–558. - PubMed
-
- Lilleri D, Fornara C, Chiesa A, Caldera D, Alessandrino EP, et al. (2008) Human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in adult allogeneic hematopoietic stem cell transplant recipients and immune control of viral infection. Haematologica 93: 248–256. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

