Deferred pre-emptive switch from calcineurin inhibitor to sirolimus leads to improvement in GFR and expansion of T regulatory cell population: a randomized, controlled trial
- PMID: 24146762
- PMCID: PMC3795731
- DOI: 10.1371/journal.pone.0075591
Deferred pre-emptive switch from calcineurin inhibitor to sirolimus leads to improvement in GFR and expansion of T regulatory cell population: a randomized, controlled trial
Abstract
Background: Measures to prevent chronic calcineurin inhibitor (CNI) toxicity have included limiting exposure by switching to sirolimus (SIR). SIR may favorably influence T regulator cell (T(reg)) population. This randomized controlled trial compares the effect of switching from CNI to SIR on glomerular filtration rate (GFR) and T(reg) frequency.
Methods: In this prospective open label randomized trial, primary living donor kidney transplant recipients on CNI-based immunosuppression were randomized to continue CNI or switched to sirolimus 2 months after surgery; 29 were randomized to receive CNI and 31 to SIR. All patients received mycophenolate mofetil and steroids. The main outcome parameter was estimated GFR (eGFR) at 180 days. T(reg) population was estimated by flowcytometry.
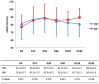
Results: Baseline characteristics in the two groups were similar. Forty-eight patients completed the trial. At six months, patients in the SIR group had significantly higher eGFR as compared to those in the CNI group (88.94 ± 11.78 vs 80.59 ± 16.51 mL/min, p = 0.038). Patients on SIR had a 12 mL/min gain of eGFR of at the end of six months. Patients in the SIR group showed significant increase in T(reg) population at 30 days, which persisted till day 180. There was no difference in the adverse events in terms of number of acute rejection episodes, death, infections, proteinuria, lipid profile, blood pressure control and hematological parameters between the two groups. Four patients taking SIR developed enthesitis. No patient left the study or switched treatment because of adverse event.
Conclusions: A deferred pre-emptive switch over from CNI to SIR safely improves renal function and T(reg) population at 6 months in living donor kidney transplant recipients. Registered in Clinical Trials Registry of India (CTRI/2011/091/000034).
Conflict of interest statement
Figures

References
-
- The Canadian Multicentre Transplant Study Group (1986) A randomized clinical trial of cyclosporine in cadaveric renal transplantation. Analysis at three years. N Engl J Med 314: 1219–1225. - PubMed
-
- Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, et al. (2003) The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333. - PubMed
-
- Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, et al. (2007) The janus face of immunosuppression - de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney Int 71: 1271–1278. - PubMed
-
- Mourad G, Vela C, Ribstein J, Mimran A (1998) Long-term improvement in renal function after cyclosporine reduction in renal transplant recipients with histologically proven chronic cyclosporine nephropathy. Transplantation 65: 661–667. - PubMed
-
- Weir MR, Blahut S, Drachenburg C, Young C, Papademitriou J, et al. (2004) Late calcineurin inhibitor withdrawal as a strategy to prevent graft loss in patients with suboptimal kidney transplant function. Am J Nephrol 24: 379–386. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

