A tuneable switch for controlling environmental degradation of bioplastics: addition of isothiazolinone to polyhydroxyalkanoates
- PMID: 24146779
- PMCID: PMC3795700
- DOI: 10.1371/journal.pone.0075817
A tuneable switch for controlling environmental degradation of bioplastics: addition of isothiazolinone to polyhydroxyalkanoates
Abstract
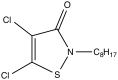
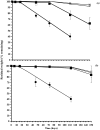
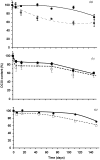
Controlling the environmental degradation of polyhydroxybutyrate (PHB) and polyhydroxyvalerate (P(HB-co-HV)) bioplastics would expand the range of their potential applications. Combining PHB and P(HB-co-HV) films with the anti-fouling agent 4,5-dichloro-2-n-octyl-4-isothiazolin-3-one (DCOI, <10% w/w) restricted microbial colonisation in soil, but did not significantly affect melting temperature or the tensile strength of films. DCOI films showed reduced biofouling and postponed the onset of weight loss by up to 100 days, a 10-fold increase compared to unmodified films where the microbial coverage was significant. In addition, the rate of PHA-DCOI weight loss, post-onset, reduced by about 150%; in contrast a recorded weight loss of only 0.05% per day for P(HB-co-HV) with a 10% DCOI loading was observed. This is in stark contrast to the unmodified PHB film, where a recorded weight loss of only 0.75% per day was made. The 'switch' that initiates film weight loss, and its subsequent reduced rate, depended on the DCOI loading to control biofouling. The control of biofouling and environmental degradation for these DCOI modified bioplastics increases their potential use in biodegradable applications.
Conflict of interest statement
Figures






Similar articles
-
Degradation of medium-chain-length polyhydroxyalkanoates in tropical forest and mangrove soils.Appl Biochem Biotechnol. 2005 Jul;126(1):23-33. doi: 10.1007/s12010-005-0003-7. Appl Biochem Biotechnol. 2005. PMID: 16014996
-
Polyhydroxyalkanoates (PHAs) degradation by the newly isolated marine Bacillus sp. JY14.Chemosphere. 2021 Nov;283:131172. doi: 10.1016/j.chemosphere.2021.131172. Epub 2021 Jun 12. Chemosphere. 2021. PMID: 34157624
-
Biodegradation Studies of Polyhydroxybutyrate and Polyhydroxybutyrate-co-Polyhydroxyvalerate Films in Soil.Int J Mol Sci. 2023 Apr 21;24(8):7638. doi: 10.3390/ijms24087638. Int J Mol Sci. 2023. PMID: 37108799 Free PMC article.
-
Bacterial synthesis of biodegradable polyhydroxyalkanoates.J Appl Microbiol. 2007 Jun;102(6):1437-49. doi: 10.1111/j.1365-2672.2007.03335.x. J Appl Microbiol. 2007. PMID: 17578408 Review.
-
Polyhydroxybutyrate: plastic made and degraded by microorganisms.Rev Environ Contam Toxicol. 1999;159:1-24. doi: 10.1007/978-1-4612-1496-0_1. Rev Environ Contam Toxicol. 1999. PMID: 9921137 Review.
Cited by
-
Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: a review.RSC Adv. 2021 May 10;11(28):17151-17196. doi: 10.1039/d1ra02390j. eCollection 2021 May 6. RSC Adv. 2021. PMID: 35479695 Free PMC article. Review.
-
Carbon Recycling of High Value Bioplastics: A Route to a Zero-Waste Future.Polymers (Basel). 2024 Jun 7;16(12):1621. doi: 10.3390/polym16121621. Polymers (Basel). 2024. PMID: 38931972 Free PMC article. Review.
-
Biodegradable Biobased Polymers: A Review of the State of the Art, Challenges, and Future Directions.Polymers (Basel). 2024 Aug 9;16(16):2262. doi: 10.3390/polym16162262. Polymers (Basel). 2024. PMID: 39204482 Free PMC article. Review.
-
The Impact of Abiotic and Biotic Conditions for Degradation Behaviors of Common Biodegradable Products in Stabilized Composts.Materials (Basel). 2024 Jun 16;17(12):2948. doi: 10.3390/ma17122948. Materials (Basel). 2024. PMID: 38930317 Free PMC article.
References
-
- Shah AA, Hasan F, Hameed A, Ahmed S (2008) Biological degradation of plastics: a comprehensive review. Biotechnol Adv 26: 246–265. - PubMed
-
- Chanprateep S (2010) Current trends in biodegradable polyhydroxyalkanoates. J Biosci Bioeng 110: 621–632. - PubMed
-
- Nagai N, Matsunobe T, Imai T (2005) Infrared analysis of depth profiles in UV-photochemical degradation of polymers. Polym Degrad Stab 88: 224–233.
-
- Rasmussen K, Grampp G, Eesbeek MV, Rohr T (2010) Thermal and UV degradation of polymer films studied in situ with ESR spectroscopy. ACS Appl Mater Interfaces 2: 1879–1883.
-
- Modelli A, Calcagno B, Scandola M (1999) Kinetics of aerobic polymer degradation in soil by means of the ASTM D 5988-96 standard method. Journal of Environ Polym Degrad 7: 109–116.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

