Expression of the B-cell receptor component CD79a on immature myeloid cells contributes to their tumor promoting effects
- PMID: 24146823
- PMCID: PMC3797715
- DOI: 10.1371/journal.pone.0076115
Expression of the B-cell receptor component CD79a on immature myeloid cells contributes to their tumor promoting effects
Abstract
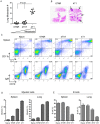
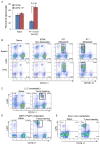
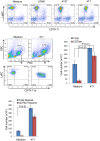
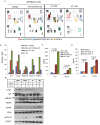
The role of myeloid derived suppressor cells (MDSCs) in promoting tumorigenesis is well-established, and significant effort is being made to further characterize surface markers on MDSCs both for better diagnosis and as potential targets for therapy. Here we show that the B cell receptor adaptor molecule CD79a is unexpectedly expressed on immature bone marrow myeloid cells, and is upregulated on MDSCs generated in multiple different mouse models of metastatic but not non-metastatic cancer. CD79a on MDSCs is upregulated and activated in response to soluble factors secreted by tumor cells. Activation of CD79a on mouse MDSCs, by crosslinking with a specific antibody, maintained their immature phenotype (CD11b+Gr1+), enhanced their migration, increased their suppressive effect on T cell proliferation, and increased secretion of pro-tumorigenic cytokines such as IL-6 and CCL22. Furthermore, crosslinking CD79a on myeloid cells activated signaling through Syk, BLNK, ERK and STAT3 phosphorylation. In vivo, CD79+ myeloid cells showed enhanced ability to promote primary tumor growth and metastasis. Finally we demonstrate that CD79a is upregulated on circulating myeloid cells from lung cancer patients, and that CD79a+ myeloid cells infiltrate human breast tumors. We propose that CD79a plays a functional role in the tumor promoting effects of myeloid cells, and may represent a novel target for cancer therapy.
Conflict of interest statement
Figures




Similar articles
-
A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model.Breast Cancer Res. 2013;15(5):R79. doi: 10.1186/bcr3473. Breast Cancer Res. 2013. PMID: 24021059 Free PMC article.
-
The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway.Immunity. 2013 Mar 21;38(3):475-88. doi: 10.1016/j.immuni.2012.11.015. Epub 2013 Feb 28. Immunity. 2013. PMID: 23453634 Free PMC article.
-
Macrophages are more potent immune suppressors ex vivo than immature myeloid-derived suppressor cells induced by metastatic murine mammary carcinomas.J Immunol. 2014 Jan 1;192(1):512-22. doi: 10.4049/jimmunol.1300096. Epub 2013 Nov 27. J Immunol. 2014. PMID: 24285836
-
Myeloid-derived suppressor cells in breast cancer.Breast Cancer Res Treat. 2013 Jul;140(1):13-21. doi: 10.1007/s10549-013-2618-7. Epub 2013 Jul 5. Breast Cancer Res Treat. 2013. PMID: 23828498 Free PMC article. Review.
-
Negative regulation of myeloid-derived suppressor cells in cancer.Immunol Invest. 2012;41(6-7):562-80. doi: 10.3109/08820139.2012.685538. Immunol Invest. 2012. PMID: 23017135 Free PMC article. Review.
Cited by
-
Self-supervised deep clustering of single-cell RNA-seq data to hierarchically detect rare cell populations.Brief Bioinform. 2023 Sep 22;24(6):bbad335. doi: 10.1093/bib/bbad335. Brief Bioinform. 2023. PMID: 37769630 Free PMC article.
-
Monoclonal Antibodies in Oncology: A Decade of Novel Options.Cell Biochem Biophys. 2023 Sep;81(3):395-408. doi: 10.1007/s12013-023-01144-1. Epub 2023 Jul 3. Cell Biochem Biophys. 2023. PMID: 37395856 Review.
-
MetastaSite: Predicting metastasis to different sites using deep learning with gene expression data.Front Mol Biosci. 2022 Jul 22;9:913602. doi: 10.3389/fmolb.2022.913602. eCollection 2022. Front Mol Biosci. 2022. PMID: 35936793 Free PMC article.
-
Resolving therapy resistance mechanisms in multiple myeloma by multiomics subclone analysis.Blood. 2023 Nov 9;142(19):1633-1646. doi: 10.1182/blood.2023019758. Blood. 2023. PMID: 37390336 Free PMC article.
-
Oncogene-independent BCR-like signaling adaptation confers drug resistance in Ph-like ALL.J Clin Invest. 2020 Jul 1;130(7):3637-3653. doi: 10.1172/JCI134424. J Clin Invest. 2020. PMID: 32191635 Free PMC article.
References
-
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, et al. (2004) Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6: 409–421. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

