Iron accumulates in Huntington's disease neurons: protection by deferoxamine
- PMID: 24146952
- PMCID: PMC3795666
- DOI: 10.1371/journal.pone.0077023
Iron accumulates in Huntington's disease neurons: protection by deferoxamine
Erratum in
- PLoS One. 2013;8(11). doi:10.1371/annotation/67f555f5-35b7-4468-8bab-26d518942803
Abstract
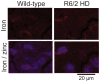
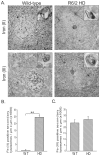
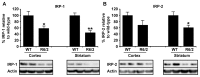
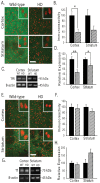
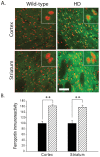
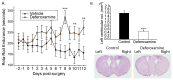
Huntington's disease (HD) is a progressive neurodegenerative disorder caused by a polyglutamine-encoding CAG expansion in the huntingtin gene. Iron accumulates in the brains of HD patients and mouse disease models. However, the cellular and subcellular sites of iron accumulation, as well as significance to disease progression are not well understood. We used independent approaches to investigate the location of brain iron accumulation. In R6/2 HD mouse brain, synchotron x-ray fluorescence analysis revealed iron accumulation as discrete puncta in the perinuclear cytoplasm of striatal neurons. Further, perfusion Turnbull's staining for ferrous iron (II) combined with transmission electron microscope ultra-structural analysis revealed increased staining in membrane bound peri-nuclear vesicles in R6/2 HD striatal neurons. Analysis of iron homeostatic proteins in R6/2 HD mice revealed decreased levels of the iron response proteins (IRPs 1 and 2) and accordingly decreased expression of iron uptake transferrin receptor (TfR) and increased levels of neuronal iron export protein ferroportin (FPN). Finally, we show that intra-ventricular delivery of the iron chelator deferoxamine results in an improvement of the motor phenotype in R6/2 HD mice. Our data supports accumulation of redox-active ferrous iron in the endocytic / lysosomal compartment in mouse HD neurons. Expression changes of IRPs, TfR and FPN are consistent with a compensatory response to an increased intra-neuronal labile iron pool leading to increased susceptibility to iron-associated oxidative stress. These findings, together with protection by deferoxamine, support a potentiating role of neuronal iron accumulation in HD.
Conflict of interest statement
Figures
References
-
- Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR et al. (2011) Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol 10: 31-42. doi:10.1016/S1474-4422(10)70276-3. PubMed: 21130037. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

