The suppressive role and aberrent promoter methylation of BTG3 in the progression of hepatocellular carcinoma
- PMID: 24147003
- PMCID: PMC3798399
- DOI: 10.1371/journal.pone.0077473
The suppressive role and aberrent promoter methylation of BTG3 in the progression of hepatocellular carcinoma
Abstract
Background: BTG3 (B-cell translocation gene 3) has been identified as a tumor suppressor and hypermethylation contributes to its down-regulation in some tumors, but its role in hepatocellular carcinoma (HCC) remain unknown. This study aimed to detect the expression and methylation status of BTG3 in HCC cell lines or tissues, and determine its function in HCC progression.
Methodology: The expression of BTG3 was detected in HCC cell lines and HCC tissue by real-time RT-PCR, Western blot or immunohistochemistry. The promoter methylation status of BTG3 was measured by using methylation-specific PCR in HCC cell lines. A series of assays were performed to evaluate the effect of BTG3 on proliferation, invasion and cell cycle transition in vitro.
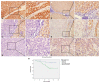
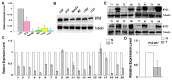
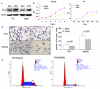
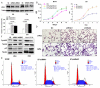
Results: BTG3 expression was lower in HCC cell lines than in hepatocyte cell line LO2 (P<0.05). BTG3 was also down-regulated in HCC tissues. Its expression was positively correlated with differentiation and distant metastasis (P<0.05). Patients with lower BTG3 expression had shorter overall survival time (P=0.029). DNA methylation directed repression of BTG3 mRNA expression in HCC cell lines. BTG3 suppressed proliferation, invasion and induces G1/S cycle arrest of HCC cells in vitro.
Conclusion: Down-regulation of BTG3 due to the promoter hypermethylation is closely associated with proliferation, invasion and cell cycle arrest of HCC cells. It may be a novel prognostic biomarker for HCC patients.
Conflict of interest statement
Figures

Similar articles
-
MicroRNA-519c-3p promotes tumor growth and metastasis of hepatocellular carcinoma by targeting BTG3.Biomed Pharmacother. 2019 Oct;118:109267. doi: 10.1016/j.biopha.2019.109267. Epub 2019 Aug 3. Biomed Pharmacother. 2019. PMID: 31387005
-
FOXC1 promotes HCC proliferation and metastasis by Upregulating DNMT3B to induce DNA Hypermethylation of CTH promoter.J Exp Clin Cancer Res. 2021 Feb 1;40(1):50. doi: 10.1186/s13046-021-01829-6. J Exp Clin Cancer Res. 2021. PMID: 33522955 Free PMC article.
-
Promoter hypomethylation up-regulates CD147 expression through increasing Sp1 binding and associates with poor prognosis in human hepatocellular carcinoma.J Cell Mol Med. 2011 Jun;15(6):1415-28. doi: 10.1111/j.1582-4934.2010.01124.x. Epub 2010 Jul 12. J Cell Mol Med. 2011. PMID: 20629990 Free PMC article.
-
Promoter hypermethylation of DNA damage response genes in hepatocellular carcinoma.Cell Biol Int. 2012 May 1;36(5):427-32. doi: 10.1042/CBI20100851. Cell Biol Int. 2012. PMID: 21864295
-
TRIM52 up-regulation in hepatocellular carcinoma cells promotes proliferation, migration and invasion through the ubiquitination of PPM1A.J Exp Clin Cancer Res. 2018 Jun 13;37(1):116. doi: 10.1186/s13046-018-0780-9. J Exp Clin Cancer Res. 2018. PMID: 29898761 Free PMC article.
Cited by
-
Hypoxia-induced downregulation of B-cell translocation gene 3 confers resistance to radiation therapy of colorectal cancer.J Cancer Res Clin Oncol. 2020 Oct;146(10):2509-2517. doi: 10.1007/s00432-020-03307-6. Epub 2020 Jul 3. J Cancer Res Clin Oncol. 2020. PMID: 32620986 Free PMC article.
-
Aberrant promoter methylation profiles and association with survival in patients with hepatocellular carcinoma.Onco Targets Ther. 2017 May 8;10:2501-2509. doi: 10.2147/OTT.S128058. eCollection 2017. Onco Targets Ther. 2017. PMID: 28507442 Free PMC article.
-
The roles of BTG3 expression in gastric cancer: a potential marker for carcinogenesis and a target molecule for gene therapy.Oncotarget. 2015 Aug 14;6(23):19841-67. doi: 10.18632/oncotarget.3734. Oncotarget. 2015. PMID: 25904053 Free PMC article.
-
The clinicopathological significances and related signal pathways of BTG3 mRNA expression in cancers: A bioinformatics analysis.Front Genet. 2022 Sep 16;13:1006582. doi: 10.3389/fgene.2022.1006582. eCollection 2022. Front Genet. 2022. PMID: 36186486 Free PMC article.
-
Direct Downregulation of B-Cell Translocation Gene 3 by microRNA-93 Is Required for Desensitizing Esophageal Cancer to Radiotherapy.Dig Dis Sci. 2017 Aug;62(8):1995-2003. doi: 10.1007/s10620-017-4579-x. Epub 2017 Apr 22. Dig Dis Sci. 2017. PMID: 28434073
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

